Synthesis of Benzothiophene
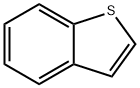
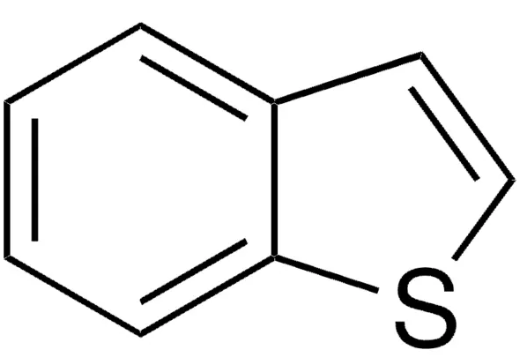
Benzothiophene is constituted by fusion of the benzene ring with the thiophene ring. There are two possible methods of fusion of the benzene ring with two different sites, namely 2,3- or [b] and 3,4- or [c] sites of the thiophene ring and accordingly two isomeric products: (1) benzo[b]thiophene and (2) benzo[c]thiophene, also known as isobenzothiophene.
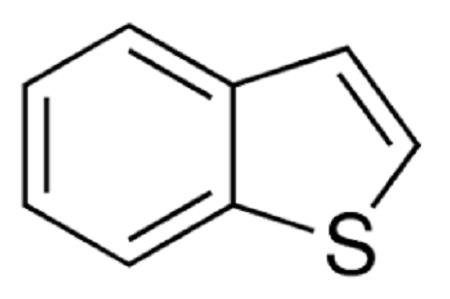
Structure
Benzo[b]thiophene is a heteroaromatic bicyclic ring system with 10π electrons in which the benzene ring is fused with the 2,3-position or [b] site of the thiophene ring. In benzothiophene, both benzene and thiophene rings are planar because all the carbon atoms are sp2 hybridized. However, substitution in either of the rings causes deviation of 1 degree in planarity. Both the C-S bonds compared to thiophene (1.714 Å) are longer, while the C2-C3 (1.370 Å) bond is shorter. Benzothiophenes being aromatic in nature undergo electrophilic substitution at position 3 unless directed to position 2 by substitution effects. The electrophilic substitution in isolated thiophene is faster compared to fused thiophene ring systems, while such substitutions in the benzene ring are comparatively slower.
The benzo[b]thiophene ring system is also distributed in various natural products, either as a substructure or isolated form. Many of them are pharmacologically very active and some of them are in clinical use for the treatment of various ailments.
Physical Properties
It is a colorless solid with an mp of 32°C and a bp of 220–221°C; it smells like naphthalene. The resonance energy of benzo[b]thiophene is 58 kcal/mol and it is quite stable at room temperature.
Synthesis
Benzothiophene has been prepared by intramolecular cyclization of various aryl sulfides in the presence of different catalysts under different reaction conditions.
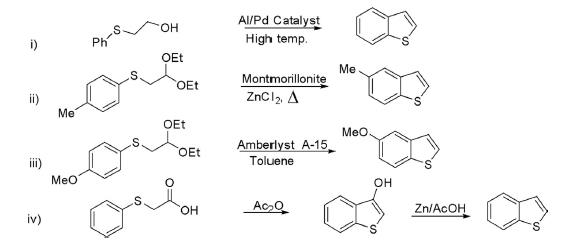
(i) Benzo[b]thiophene has been prepared by oxidation-cyclization of 2-phenylthioethanol in the presence of Pd/Al as catalyst at high temperature.
(ii) Arylmercapto acetals have also been used as a precursor for the construction of benzo[b]thiophene through gas phase reaction using ZnCl2-impregnated montmorillonite as catalyst.
(iii) Arylmercapto acetals are also cyclized using Amberlyst A-15 as catalyst in boiling toluene as shown in the foregoing scheme.
(iv) Arylthioacetic acid obtainable from the reaction of thiophenol and chloroacetic acid in refluxing alcohol followed by cyclization in acetic anhydride gave 3-hydroxybenzo[b]thiophene, which on dehydroxylation afforded benzo[b]thiophene.
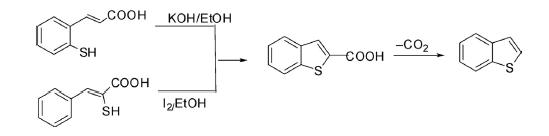
Oxidative cyclization of 2-mercaptocinnamic acid in an alkaline solution of K3Fe(CN)6 afforded benzo[b]thiophene. Alternatively, it has also been prepared by oxidative cyclization of α-mercaptocinnamic acid by iodine.
You may like
Related articles And Qustion
See also
Lastest Price from Thianaphthene manufacturers
US $0.00-0.00/kg2025-11-20
- CAS:
- 95-15-8
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 100tons

US $10.00/KG2025-04-21
- CAS:
- 95-15-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt