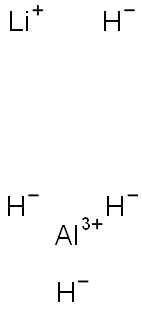Uses of Lithium aluminium hydride in organic chemistry
Lithium aluminium hydride (LiAl H4), commonly abbreviated to LAH, is a powerful reducing agent used in organic chemistry. It is more powerful than the related reducing agent sodium borohydride due to the weaker Al-H bond compared to the B-H bond. It will convert esters, carboxylic acids and ketones to alcohols; and nitro compounds into amines.
LAH violently reacts with water, including atmospheric moisture, and the pure material is pyrophoric. Commercial material is inhibited with mineral oil to allow handling in air.
Pure, recrystallized (from diethyl ether) LAH is a white solid. Commercial samples are almost always grey due to trace contamination with aluminium metal. White air-exposed commercial samples of LAH have absorbed enough moisture to become a mixture of lithium hydroxide and aluminium hydroxide.
Use in organic chemistry
Lithium aluminium hydride is widely used in organic chemistry as a very powerful reducing agent. Despite handling problems associated with its reactivity, it is even used at the small-industrial scale, although for large scale reductions the related reagent sodium bis(2-methoxyethoxy)aluminium hydride or Red-Al is more usual. For such purposes it is usually used in solution in diethyl ether, and an aqueous workup is usually performed after the reduction in order to remove inorganic by-products. It is most commonly used for the reduction of esters and carboxylic acids to primary alcohols; prior to the advent of LiAlH4 this was a difficult conversion involving sodium metal in boiling ethanol (the Bouveault-Blanc reduction). Aldehydes and ketones can also be reduced to alcohols by LAH, but this is usually done using milder reagents such as NaBH4. α,β-Unsaturated ketones are reduced to allylic alcohols. When epoxides are reduced using LAH, the reagent attacks the less hindered end of the epoxide, usually producing a secondary or tertiary alcohol. It reduces by progressive breakup of the complex AlH4− and transfer of hydride ions to the positive centre in an organic compound which may have a low density of electrons due to inductive or mesomeric effects.
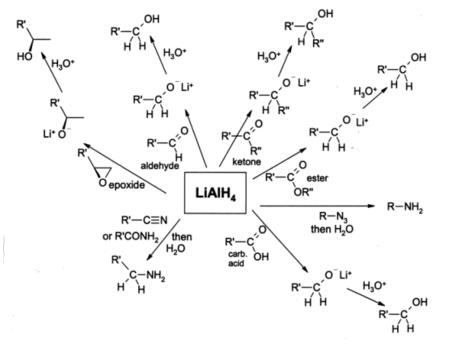
Amines can be prepared by the LAH reduction of amides , oximes , nitriles, nitro compounds or alkyl azides. LAH is also able to reduce primary alkyl halides to alkanes.
Lithium aluminium hydride is not able to reduce simple alkenes or benzene rings, and alkynes are only reduced if an alcohol group is nearby.
Related articles And Qustion
Lastest Price from Lithium Aluminum Hydride manufacturers

US $10.00/kg2025-04-21
- CAS:
- 16853-85-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1000kg

US $0.00-0.00/kg2025-04-21
- CAS:
- 16853-85-3
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 20 tons