Lithium Aluminum Hydride: Lithium Storage Mechanism and its Synthesis Procedure
General Description
Lithium Aluminum Hydride, a versatile reagent in organic chemistry, is known for its ability to reduce various functional groups. In lithium storage for batteries, XRD analysis reveals a complex mechanism involving phases like Lithium Aluminum Hydride, LiH, metallic Al, and LiAl. Side reactions with electrolytes form new phases and byproducts. The synthesis of Lithium Aluminum Hydride through ball milling is crucial for its purity and electrochemical performance. Evaluation shows a specific capacity of 1729 mAh/g but highlights the need for improved cycling stability. Overall, Lithium Aluminum Hydride's diverse applications and intricate lithium storage behavior underscore its significance in various industries.1

Figure 1. Lithium Aluminum Hydride
Lithium Storage Mechanism
The electrochemical properties of the Li3AlH6 are evaluated as an anode material for Li-ion batteries. The electrochemical lithium storage behavior of the Li3AlH6 is first studied by cyclic voltammetry (CV). The representative CV curves of the first three cycles of the electrode in a voltage window of 0.005–3.0 V (vs. Li/Li+) was presented in Fig. 2a. In the first cathodic scan, the irreversible electrochemical reduction reactions between Li3AlH6 and Li+ occurs accompanying the formation of LiH and LiAl (Li3AlH6+4Li++4e−→6LiH + LiAl). An obvious anodic peak at about 0.58 V can be assigned to the Li+ extraction reaction. It is noteworthy that compared with the first cycle, the main cathodic peaks shift to ∼0.33 V in the subsequent two cycles, while the anodic peaks show negligible change. This phenomenon further confirms the above electrochemical lithium storage in Li3AlH6 (Li3AlH6+4Li++4e−→6LiH + LiAl↔6LiH + Al+Li++e−), which corresponds to the XRD result. The stability performance is another prominent concern for an advanced anode material in Li-ion batteries. Fig. 2b illustrates the charge capacity of as-prepared Li3AlH6 as a function of cycle number. The discharge capacity of Li3AlH6 decreased dramatically from 1729 mAh/g at the first cycle to 509 mAh/g at the second cycle due to the partial reversibility of lithium storage in Li3AlH6. Fortunately, the as-prepared Li3AlH6 exhibits an approving cycling stability after the second cycle. The slight decay in the discharge capacity with 362 mAh/g after 20 cycles, realizing 71 % capacity retention relative to the second cycle. Compared with the bulk Al electrode with only 30 % capacity retention after 10 cycles, the Li3AlH6 electrode possesses an outstanding stability. Therefore, LiH that formed in the initial stage of the lithiation of Li3AlH6 acts as an inactive material, however, it as a volumetric expansion buffer plays an important role in relieving volume expansion in delithiation/lithiation process. On the other hand, the unknown phases from the partial reaction of Li3AlH6 with electrolytes may play a similar role with the solid electrolyte interface (SEI) layer.2
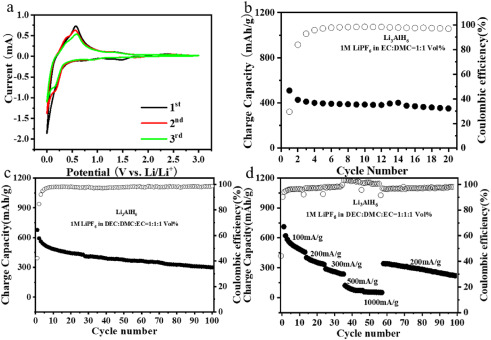
Figure 2. Storage Behavior of the Lithium Aluminum Hydride
Synthesis Procedure
Li3AlH6 was synthesized by milling LiAlH4 and LiH with a molar ratio of 1:2 under 1.0 bar argon (Ar) for 24 h on a planetary ball mill rotating at 500 rpm. The ball-to-sample weigh ratio was about 60:1. The electrode materials of Li3AlH6 was prepared by milling active materials with acetylene black with weight ratios of 85:15, applied a planetary ball mill with a rotating speed of 350 rpm for 12 h. To prevent contamination from moisture and air, all sample handlings were carried out in a glovebox filled with purified argon (H2O: <1 ppm, O2: <1 ppm). 2
References:
[1] LIANG C, YE Z, YANG Y, et al. Lithium aluminum hydride Li3AlH6: new insight into the anode material for liquid-state lithium-ion batteries[J]. Heliyon, 2023, 6 1: 184-188. DOI:10.1016/j.heliyon.2023.e21765.[2] LIANG C, YE Z, YANG Y, et al. Lithium aluminum hydride Li3AlH6: new insight into the anode material for liquid-state lithium-ion batteries[J]. Heliyon, 2023, 6 1: 184-188. DOI:10.1016/j.heliyon.2023.e21765.
You may like
Related articles And Qustion
See also
Lastest Price from Lithium Aluminum Hydride manufacturers

US $10.00/kg2025-04-21
- CAS:
- 16853-85-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1000kg

US $0.00-0.00/kg2025-04-21
- CAS:
- 16853-85-3
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 20 tons