Synthesis of Benzothiazole
Benzothiazole is a conjugated sp2-hybridized bicyclic 10π electron heteroaromatic, constituted by fusion of benzene with the 4,5-positions of the thiazole ring. 2-Substituted benzothiazoles such as 2-chloro- and 2-phenylbenzothiazoles were first reported by A.W. Hofmann in 1879, while use of 2-sulfanylbenzothiazoles as vulcanization accelerators for natural and synthetic rubber was discovered in 1921.
The benzothiazole ring is an important scaffold in drug discovery because of its diverse pharmacological activities. This ring system is present in numerous marine and terrestrial plants with a wide range of pharmacological activities.
Parent benzothiazole was first isolated in 1967 from the volatiles of American cranberries Vaccinium macrocarpon Ait. var. Early Black. 6-Hydroxybenzothiazole-5-acetic acid, known as antibiotic C304A or M4582, is a naturally occurring benzothiazole.
Physical Properties
Benzothiazole is a colorless liquid with a bp of 227°C and an mp of 2°C. It is sparingly soluble in water, soluble in alcohols and carbon disulfide, and highly soluble in ether and acetone. Benzothiazole is a weaker base (pKa 1.2) than thiazole (pKa 2.52) with a density of 1.246 g/cm3.
Synthesis
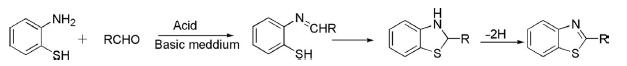
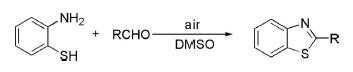
1. Condensation of 2-aminothiophenol with aromatic or aliphatic aldehyde in an acidic or basic medium initially gave Schiff's base, which cyclized by dehydrogenation to deliver 2-substituted benzothiazole.
2. Recently, a straightforward synthesis of 2-aryl benzothiazole has been reported from the reaction of 2-aminothiophenol with aryl aldehyde in air/DMSO without the use of any catalyst and afforded benzothiazole in excellent yields.
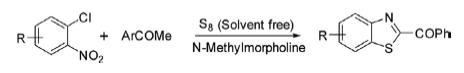
By using samarium triflate as a reusable catalyst in lieu of air/DMSO afforded 2-substituted benzothiazole under very mild reaction conditions in good yields. A wide range of 2-aroylbenzothiazoles have been synthesized in high yields by heating a mixture of 2-halonitrobenzene, acetophenone, and elemental sulfur in the presence of N-methylmorpholine under solvent-free conditions.
Applications
The benzothiazole ring is an important scaffold for the preparation of dyes used in the identification of lanthanide metal ions in aqueous media. The most important industrial applications of benzothiazole derivatives are recognized as corrosion inhibitors and surface-active chelating agents for mineral processing. They also exhibit antioxidant properties. Some benzothiazole derivatives are highly useful as insecticides and herbicides. Riluzole, 6-(trifluoromethoxy)benzothiazol-2-amine, in clinical use has anticonvulsant and neuroprotective properties. Some of the benzothiazoline derivatives such as 3-alkyl- or 3-arylbenzothiazolines have displayed very promising antiinflammatory and antibacterial properties and are used as drugs. 2-(Halophenyl)benzothiazoles and 3,4-disubtituted benzothiazol-2(3H)-ones have exhibited anthelmintic and fungicidal properties.
Lastest Price from Benzothiazole manufacturers

US $0.00/kg2025-09-29
- CAS:
- 95-16-9
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $10.00/KG2025-04-21
- CAS:
- 95-16-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100 mt