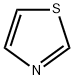
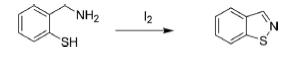
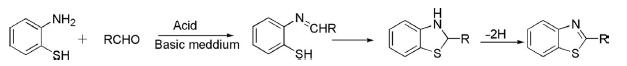
Synthesis of Thiazole
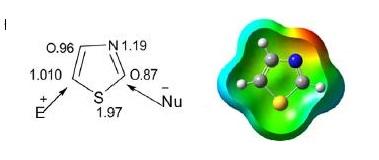
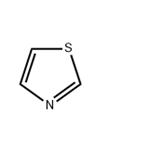
Thiazole is a five-membered, unsaturated, planar, π-excessive heteroaromatic containing one sulfur atom and one pyridine-type nitrogen atom at position 3 of the cyclic ring system. It is also called 1,3-thiazole. The higher aromaticity of thiazole is due to delocalization of a lone pair of sulfur electrons across the ring, which is evidenced by chemical shifts of ring hydrogen at δ 7.27 and 8.77 ppm (C2 and C4), indicating diamagnetic ring current. The calculated π electron density revealed that C5 is the primary site followed by C4 for electrophilic substitution, whereas C2 is the preferred site for nucleophilic substitution.
There are numerous natural products that possess a thiazole ring with broad pharmacological activities. Thiamine, also known as vitamin B1, possesses a thiazole ring linked with 2-methylpyrimidine-4-amine as hydrochloride salt. Bacillamide A (1) known as algaecide and its two other analogs, bacillamide B (2) and bacillamide C (3), are isolated from Bacillus endophyticus but did not display antibiotic activity. The related compound neobacillamide A (4), together with bacillamide C, is isolated from Bacillus vallismortis C89 associated with the sponge Dysidea avara. It is cytotoxic against HL60 human leukemia and lung cancer cells.
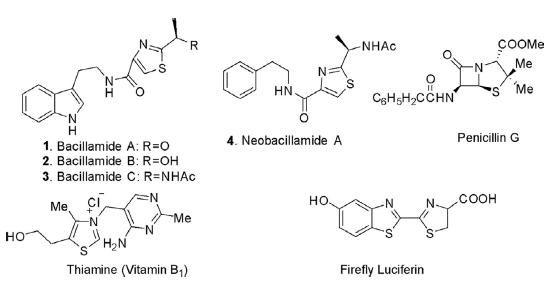
Thiazole is one of the important scaffolds in heterocyclic chemistry and diversely present in pharmacologically active compounds of synthetic and natural product origins. Thiazole is a versatile building block for the construction and lead generation of new drug discoveries. Numerous diazole-based compounds are in clinical use as anticancer, antileukemic, antiinflammatory, antiviral, antifungal, antirheumatic, immunomodulator, and antiparasitic agents.
Physical Properties
Thiazole is a pale-yellow flammable, with a pyridine-like odor, a bp of 116–118°C, and a pKa of 2.5 (conjugate acid). Its density is 1.2 gm/cm3, and it is fairly soluble in ether and alcohol but sparingly soluble in water. The ionization potential is 9.50 eV and its dipole moment is 1.61 D.
Chemical Reactivity
Thiazole is more basic than oxazole but less basic than pyridine and forms stable picrate salts. Its chemical reactivity is very similar to thiophene and pyridine due to the presence of thiophene-type sulfur at position 1 and pyridine-type nitrogen at position 3 of the thiazole ring. There are three possible sites, sulfur, nitrogen, and C5, for electrophilic reactions. However, electronically poor site C2 is prone to nucleophilic attack.
Synthesis
There are numerous protocols for construction of the thiazole ring system but only broadly used synthetic routes are mentioned here.
1. Hantzsch's Synthesis
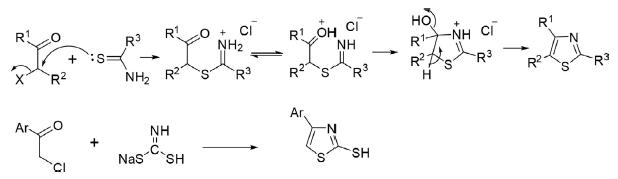
Hantzsch reported the first synthesis of thiazole in 1887 by the reaction of α-haloketone or aldehyde with thioamide. Ammonium dithiocarbonate is also used in lieu of thioamides for the synthesis of thiazole derivatives.
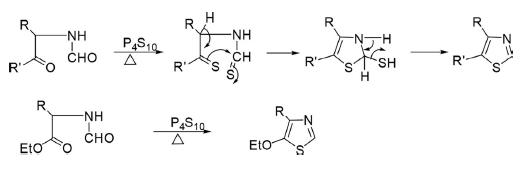
2. Gabriel Synthesis
Parent and 2,5-disubstituted thiazoles have been prepared from the reaction of α-acylamino ketones or amino acetal on reaction with phosphorous pentasulfide in good yields.
You may like
Lastest Price from Thiazole manufacturers

US $0.00-0.00/KG2025-06-26
- CAS:
- 288-47-1
- Min. Order:
- 1KG
- Purity:
- 99.0%
- Supply Ability:
- 10000KGS
US $0.00-0.00/kg2025-04-04
- CAS:
- 288-47-1
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1Ton