Introduction of Triphosgene
General description
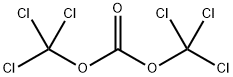
Triphosgene is also known as solid phosgene. Its chemical name is bis (Trichloromethyl) carbonate, and its English name is bisgriehloromethyl) carbonate or triphosgene, abbreviated as BTC. Triphosgene is a white crystal, similar to the smell of phosgene. It is mainly used to synthesize chloroformate, isocyanate, polycarbonate and acyl chloride. It is widely used as an intermediate in plastics, medicine, herbicides and pesticides.
Application and pharmacology
1.Triphosgene as Mild Reagents for Chemoselective Dehydration of Tertiary Alcohols. The utility of triphosgene and DMAP as mild reagents for chemoselective dehydration of tertiary alcohols is reported. Performed in dichloromethane at room temperature, this reaction is readily tolerated by a broad scope of substrates, yielding alkenes preferentially with the (E)-geometry. While formation of the Hofmann products is generally favored, a dramatic change in alkene selectivity toward the Zaitzev products is observed when the reaction is carried out in dichloroethane at reflux.[1] In recent years, our group has developed methods to chlorinate primary and secondary alcohols using a mixture of triphosgene and amine bases, particularly triethylamine or pyridine.10Recognizing that SOCl2or POCl3also serve as reagents for the chlorination of alcohols, we hypothesized that our triphosgene-amine base chemistries could be perhaps applied to dehydrate tertiary alcohols.
Figure 1 Triphosgene as Mild Reagents for Chemoselective Dehydration of Tertiary Alcohols
2. As highly toxic and accessible chemical reagents, phosgene and triphosgene have become serious threat to public safety. So, it is highly desirable to develop facile methods to detect and recognize them. In this Article, a novel fluorescent chemosensor, Phos-4, has been constructed with 1,8-naphthalimide as the fluorophore and 2-(2-aminophenyl)imidazol as the recognition sites for discrimination between phosgene and triphosgene in dual-channel mode for the first time. Owing to the difference in electrophilicity between chlorocarbonyl and trichloromethoxycarbonyl, the sensing reaction of Phos-4 with phosgene undergoes twice carbamylations to afford a cyclic product with green fluorescence, and only once carbamylation occurs for triphosgene to form non-cyclic product with blue fluorescence. The sensor Phos-4 exhibits high sensitivity (the limit of detection, 3.2 nM for phosgene and 1.9 nM for triphosgene) and high selectivity in solutions. Furthermore, a facile test papers containing Phos-4-embedded nanofibrous membrane have been fabricated by the electrospinning technology. The test papers can provide visual and selective detection of phosgene with a lower limit of detection (42 ppb) and a faster response ( 10 s) in gas phase over those in solutions. The test paper with Phos-4 is promising to be a practical detection tool of gaseous phosgene.[2]
Synthesis
Under normal conditions, triphosgene has stable chemical properties and no significant toxicity. It can successfully replace phosgene to achieve the goal of organic synthesis in many photogasification reactions. Compared with other reaction reagents, triphosgene also has more advantages. For example, it acts close to the equivalent of reactants, the reaction conditions are relatively mild, and the purity and yield of the synthetic product are significantly improved. However, when the temperature of Triphosgene is higher than 130 ° C, it will decompose slightly to produce phosgene; After moisture absorption, triphosgene will begin to decompose at 90 ° C to produce phosgene; Some impurities (such as organic amines) catalyze the decomposition of triphosgene, which will decompose rapidly at low temperature to produce phosgene; Triphosgene will be cracked into phosgene, carbon dioxide and carbon tetrachloride at high temperature.
1. There are three traditional synthesis methods of propionyl chloride. They are phosphorus trichloride method, benzoyl chloride method and phosgene method; The new process is mainly triphosgene process.[3]
Figure 2 Study on Synthesis of propionyl chloride by triphosgene method
2. Triphosgene was used as cyclizing reagent and triethylamine as base Benzoazolone, benzimidazolone and 1 were synthesized from o-aminophenol, o-phenylenediamine and catechol respectively 3-benzodioxolane-2-one. The structure of the compound was confirmed by IR, MS and NMR. The synthetic method has the characteristics of simple operation and high purity.
Toxicity and Safety
As a common production raw material for chemical enterprises, Sanguang gas is not listed in the list of hazardous chemicals, and its risk is not familiar and understood by many users, which often leads to decomposition and poisoning accidents. Therefore, it is of great practical significance to study the hazards and safety measures in the use of Triphosgene. Based on the characteristic identification of triphosgene, the hazards in the process of dissolution and reaction are identified and analyzed, and the corresponding safety measures in the use of Triphosgene are put forward according to the results of hazard identification and analysis. Triphosgene has gradually become the raw material for acyl chlorination in many enterprises because it is a non hazardous chemical, relatively stable material properties, easy transportation and easy treatment of tail gas. However, if the safety measures are not appropriate during the transportation, storage and use of triphosgene, it is very easy to cause poisoning accidents.
Triphosgene is usually dissolved in organic solvent first, and configured as a mixed solution of Triphosgene and organic solvent as reaction raw material. There is a large amount of Triphosgene in the dissolution process. Once decomposition occurs, the accident consequences will be even more serious than the photo gasification reaction. The following points should be paid attention to temperature control the triphosgene dissolving kettle is generally heated by hot water bath, and the hot water source is generally obtained by steam heating. If the hot water system is not provided with temperature display and automatic control interlocking measures, the hot water temperature is too high, which may lead to the high hot water temperature of the jacket of the dissolving kettle, Finally, triphosgene is decomposed to produce highly toxic gases such as phosgene. Purity of materials before feeding, if the purity of materials such as solvents is not detected, there are impurities (such as organic amines) that are easy to lead to the decomposition of triphosgene, which may also lead to the decomposition of Triphosgene. In addition, if there is a vacuum system in the dissolution system, material channeling in the vacuum system may also cause other impurities to enter the dissolution kettle. For solvent recovery involving triphosgene reaction unit, if there may be organic amines in the solvent, try not to apply it. If the application is considered, the organic amine content in the solvent must be controlled.[4]
Reference
1.Ganiu M. O., Cleveland A. H. & Paul J. L. et al., "Triphosgene and DMAP as Mild Reagents for Chemoselective Dehydration of Tertiary Alcohols," Organic Letters, Vol.21, No.14(2019), pp.5611-5615.
2.Wang S., Li C. & Song Q., "Fluorescent Chemosensor for Dual-Channel Discrimination between Phosgene and Triphosgene," Analytical Chemistry, Vol.91, No.9(2019), pp.5690-5697.
3.Zhu Congwei, Mo Weimin, Shen Zhenlu, et al. "Study of Triphosgene in carbonylation cyclization reaction", chemical reagent, 2006, No. 07, pp. 421-422.
4.Zhang Shaofeng: risk analysis and safety measures during the use of triphosgene, Hangzhou, Zhejiang, China, 2014.
You may like
Related articles And Qustion
Lastest Price from Triphosgene manufacturers

US $10.00/kg2025-04-21
- CAS:
- 32315-10-9
- Min. Order:
- 1kg
- Purity:
- 99.5%
- Supply Ability:
- 100 TON

US $0.00/KG2025-04-21
- CAS:
- 32315-10-9
- Min. Order:
- 1KG
- Purity:
- ≥99.5%
- Supply Ability:
- 2000mt/year