Synthesis and Uses of Benzo[b]furan
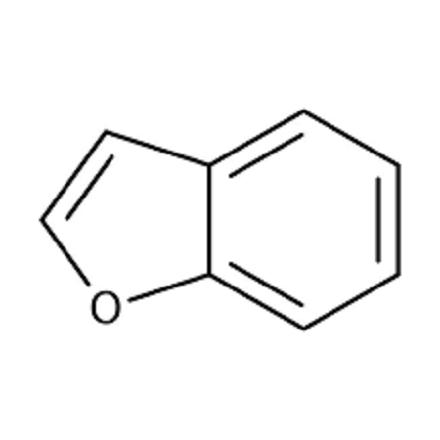
Benzo[b]furan is one of the most important heterocyclic ring systems, generated by fusion of benzene with 2,3-positions of the furan ring. The resulting bicyclic ring system is heteroaromatic with a 10π electron system in which all the atoms are sp2 hybridized, creating a planar structure. The p-orbital on each carbon is perpendicular to the plane of the ring. Participation of an electron pair of the p-orbital of oxygen in the sextet acquires considerable positive charge and accordingly the same amount of negative charge is displayed on all the ring carbon atoms of furan. The other lone pair of electrons remains in the hybrid orbital.
Physical Properties
Benzo[b]furan is a colorless, oily and water-insoluble liquid with a bp of 173°C. The 1 H NMR of benzo[b]furan in acetone d6 [δ (ppm)] due to various protons are: C2 –H, 7.79; C3 –H, 6.77; C4 –H, 7.64; C5 –H, 7.23; C6 –H, 7.30; C7 –H, 7.52. It is evident from the data that C4 –H and C7 –H protons resonated down field compared to C5 –H and C6 –H protons.
Uses
The benzofuran nucleus is frequently present in various natural products in substructural form. This class of heterocycles are multifaceted building blocks for use in optical brighteners, material science, and numerous synthetic pharmaceuticals. It was first isolated by Kraemer and Spieker from coal tar. The therapeutic importance of this class of compounds aroused considerable interest to develop the chemistry of benzo[b]furans. Almost 30 US Food and Drug Administration-approved drugs from this class of compounds either from natural or synthetic origins are already in clinical use for the treatment of various ailments. Some of the important drugs in clinical use are listed in the following scheme.
Synthesis
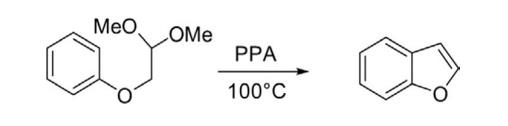
Synthesis From Aryl Ethers (a) Cyclodehydration of phenoxyacetaldehyde dimethylacetal on heating in the presence of dehydrating agents such as H2 SO4 , POCl3 , or polyphosphoric acid at 100°C provided parent benzo[b]furan.
![271-89-6 Benzo[b]furanPhysical PropertiesUsesSynthesis](https://www.chemicalbook.com/CAS/GIF/271-89-6.gif)
