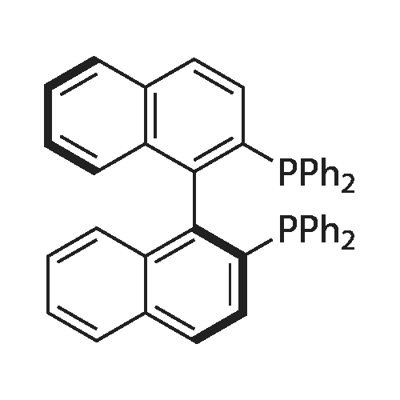
Synthesis and Preparation of (S)-(-)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl
The crystal structure of (S)-(-)-BINAP] or C44H32P2, is enantiomorphous to the previously reported (R)-(+)-BINAP , with effectively no differences in the molecular geometry apart from being of opposite absolute configuration. (S)-(-)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl crystallizes in the monoclinic space group P21, with one molecule in the asymmetric unit, as depicted as shown in the following figure. Individual molecules of (S)-(-)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl are spatially arranged in close packing along the a direction . The structures of the R and S enantiomers are essentially the same, except for being of opposite absolute configuration.
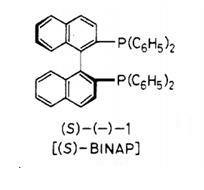
Recently numerous chiral di-terf-phosphines have been devised as ligands for transition-metal-catalyzed asymmetric syntheses in the homogeneous phase. Some years ago, Hidemasa Takaya reported (S)-2,2'-bis(diphenylphosphino)-1,1'-binaphthyl (1) (abbreviated to BINAP), a new atropisomeric bis(triaryI)phosphine. By virtue of the C2 chirality, molecular pliancy, and electronic characteristics, BINAP exhibits excellent chiral recognition ability in various asymmetric reactions and is now becoming, among others, one of the most important phosphine ligands. The BINAP-coordinated Rh(I) complexes have been shown to be efficient catalysts for asymmetric hydrogenations of α-acylaminoacrylic acids and allylic alcohols.5 Furthermore, the chiral complexes effect a remarkable enantioselective 1,3-hydrogen shift of allylamines to optically active enamines, which plays a key role in the recently established industrial synthesis of (-)-menthol. In addition, the unique features of BINAP ligands also provide advantages in examining mechanisms of transition-metal-catalyzed reactions. We initially obtained this useful diphosphine ligand, or (S)-l, by synthesis of racemic 1 followed by optical resolution with (+)-bis(μ-chloro)bis[(S)-N,N-dimethyl-l-phenylethylamine-2C,N| dipalladium(II).
Recently Murdoch reported the stereospecific synthesis of 1 and its derivatives starting from optically pure 2,2’-diamino-1,1’-binaphthyl via the optically active dibromide. We now report a new, practical route to optically pure BINAP and its derivatives which enables us to obtain various BINAP ligands in large quantities. The new procedure stems on the preparation of racemic dioxides of BINAP and its derivatives followed by optical resolution by use of readily available optically active organic acids.
References
[1]Jones M D, Paz F A A, Davies J E, et al. ( S )-(−)-2,2′-Bis¬(di¬phenyl-phosphino)-1,1′-bi¬naphthyl, a versatile chelating ligand[J]. Acta Crystallographica, 2003, 59(4):o535–o537.
[2]Dongwei Cai, Joseph F. Payack, Dean R. Bender,等. (R)-(+)- and (S)-(−)-2,2′-Bis(Diphenylphosphino)-1,1′-Binaphthyl (BINAP)[J]. Organic Syntheses, 2004, 76:6-10.
[3]Takaya H , Mashima K , Koyano K , et al. Practical synthesis of (R)- or (S)-2,2'-bis(diarylphosphino)-1,1'-binaphthyls (BINAPs)[J]. Cheminform, 1986, 17(36):864-877.
You may like
Related articles And Qustion
Lastest Price from (S)-(-)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl manufacturers

US $79.00-38.00/kg2025-04-21
- CAS:
- 76189-56-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20ton

US $0.00/KG2025-04-15
- CAS:
- 76189-56-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg