(S)-(-)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl: properties, applications and safety
General Description
(S)-(-)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl, or BINAP, is a chiral diphosphine ligand that displays excellent coordinating ability and stereoselectivity, making it highly valuable in asymmetric catalysis. Its rigid binaphthyl backbone allows for precise spatial arrangement of reactants and catalysts, promoting efficient discrimination between substrates and the formation of desired stereoisomers. BINAP also possesses good solubility and stability under a wide range of conditions, enabling its use in diverse synthetic methodologies. However, safety precautions are crucial when working with BINAP as it is toxic to aquatic life and can cause skin, eye, and respiratory irritation. Proper disposal and adherence to regulatory guidelines are necessary to safeguard both individuals and the environment. (S)-(-)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl and its derivatives show potential in the development of copper-based complexes with photocatalytic and biomedical applications, particularly in the field of cancer research.

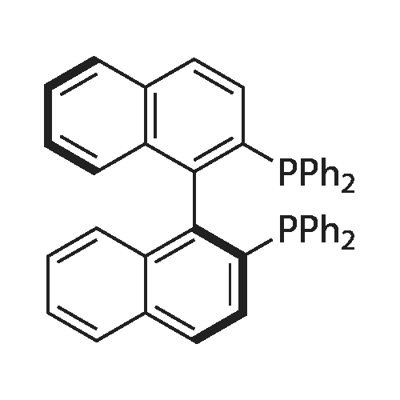
Figure 1. (S)-(-)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl
Properties
(S)-(-)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl is a chiral diphosphine ligand that has found extensive applications in asymmetric catalysis. It possesses unique properties that make it highly valuable in various chemical transformations. Firstly, BINAP exhibits excellent coordinating ability due to the presence of two phosphine groups. This allows it to form stable complexes with transition metals, enhancing the catalytic efficiency of metal-mediated reactions. The chiral nature of BINAP enables it to induce chirality in the reaction products, leading to the synthesis of enantiomerically pure compounds. Secondly, BINAP demonstrates exceptional stereoselectivity, which is crucial in asymmetric catalysis. Its rigid binaphthyl backbone imparts a high degree of steric control, enabling precise spatial arrangement of reactants and catalysts. This results in efficient discrimination between different substrates, promoting the formation of desired stereoisomers. Furthermore, BINAP possesses good solubility in common organic solvents, facilitating its use in various reaction systems. Its stability under a wide range of reaction conditions ensures its applicability in diverse synthetic methodologies. Overall, (S)-(-)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl is a versatile chiral ligand that plays a crucial role in asymmetric catalysis. 1
Applications
(S)-(-)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl finds applications in the synthesis of heteroleptic copper-based complexes incorporating π-extended diimine ligands. These complexes have been studied for their catalytic and biological activities. The copper-based photocatalysts of the type Cu(NN)(BINAP)BF4 were synthesized and evaluated for their performance in various photocatalytic processes. It was observed that they exhibited acceptable levels of activity in single electron transfer (SET) processes but showed negligible activity in proton-coupled electron transfer (PCET) or electron transfer (ET) processes. However, by modifying the ligands, suitable activity in ET processes could be restored. Furthermore, the BINAP-derived complexes were tested for their activity against triple-negative breast cancer cell lines. The results indicated that it was the copper complexes themselves, rather than the ligands, that were responsible for the observed activity. In particular, a homoleptic complex Cu(dppz)2BF4 displayed promising activity against the cancer cell lines. These findings highlight the potential of (S)-(-)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl and its derivatives in the development of copper-based complexes with photocatalytic and biomedical applications, particularly in the field of cancer research. 2
Safety
(S)-(-)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl requires careful handling due to its potential hazards. Safety precautions when working with BINAP include wearing protective clothing, such as gloves and lab coats, to minimize skin irritation. Suitable eye protection, like safety goggles, should be worn to prevent eye irritation. Working in well-ventilated areas or using respiratory protective equipment, such as masks, is necessary to reduce the risk of respiratory irritation. BINAP is toxic to aquatic life and can have long-lasting effects on ecosystems. Disposal of BINAP and its waste must adhere to local environmental regulations to prevent water contamination. Even low concentrations of BINAP can harm aquatic organisms, affecting their survival, growth, and reproduction. In summary, safety measures for working with (S)-(-)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl involve preventing skin and eye irritation, minimizing respiratory irritation, and ensuring proper disposal to protect aquatic life. Adhering to these precautions and following regulatory guidelines is crucial to ensure the well-being of individuals and safeguard the environment. 3
Reference
1. Faller JW, Wilt JC. Palladium/BINAP(S)-catalyzed asymmetric allylic amination. Org Lett. 2005 Feb 17;7(4):633-636.
2. Cruché C, Gupta S, Kodanko J, Collins SK. Heteroleptic Copper(I)-Based Complexes Incorporating BINAP and π-Extended Diimines: Synthesis, Catalysis and Biological Applications. Molecules. 2022 Jun 10;27(12):3745.
3. (S)-(-)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl. European Chemicals Agency, EC / List no. 616-305-2.
You may like
Related articles And Qustion
See also
Lastest Price from (S)-(-)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl manufacturers

US $79.00-38.00/kg2025-04-21
- CAS:
- 76189-56-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20ton

US $0.00/KG2025-04-15
- CAS:
- 76189-56-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg