Synthesis and Biochemical Effects of Xylazine
General description
Xylazine (C12H16N2S) chemical name is xylazine thiazide, also known as longpeng, is an α2-adrenoceptor agonist, with potent analgesic, sedative, muscle relaxant and anticonvulsant effects. , especially for excited and aggressive dogs. When used alone, it can be used for some simple treatment or clinical examination of dogs, such as wound treatment, bandaging, suture, etc., and is more widely used for compound anesthesia with other drugs, which can improve the anesthetic effect of other drugs and reduce its own toxic and side effects.
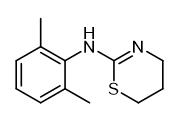
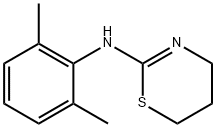
Fig. 1 The structure of Xylazine.
Synthetic routes
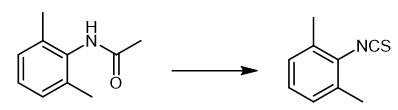
Fig. 2 The synthetic step 1 of Xylazine.
In a 500 ml clean and dry there-necked bottle, add 80 ml of anhydrous tetrahydrofuran, 80 ml of anhydrous toluene, 16.3 g of acetyl-2,6-dimethylaniline, 5 g of 50% sodium hydride, dropwise add 16 g of carbon disulfide, dropwise After stirring for 0.5 hours, poured into 1 times the amount of ice water for hydrolysis for 1 hour, and then left to stand for stratification. The toluene layer was mainly composed of 2,6-dimethylphenyl isothiocyanate [1].
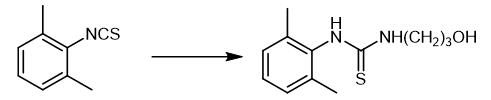
Fig. 3 The synthetic step 2 of Xylazine.
After the toluene layer organic matter obtained in the previous step was dried, deliquored and concentrated, 16 grams of 3-aminopropanols were added, and the reaction was stirred for 1 to 3 hours at 50 to 80°C. After the reaction, 1-(2, 6-Dimethyl)phenyl-3-propanolyl thiourea [1].
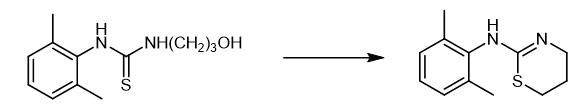
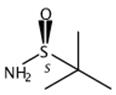
Fig. 3 The synthetic step 3 of Xylazine.
After the above-mentioned liquid was cooled a little, 160 mL of concentrated hydrochloric acid were added, and the mixture was heated to 80 to 95 °C. The reaction was stirred for 1 to 3 hours, and the reaction was completed and cooled to room temperature. Let stand for stratification, add 5 g of activated carbon to the water layer for decolorization and filtration, adjust the pH to 8 to 12 with 20% liquid caustic soda, separate out off-white solids, filter and wash with water, and recrystallize with ethanol to obtain a white crystalline powder 2,6-dimethylaniline 42 g of base thiazine, yield is 95%, MP136-138 ℃ (document value is 140~141 ℃) [1].
Biochemical Effects
In order to clarify the mechanism of xylazine-induced GH release, we investigated the effects of atipamezole, a selective alpha(2)-adrenergic antagonist, and somatostatin (SRIF) on xylazine-stimulated GH release in calves. Xylazine injection (0.30 mg/kg BW, iv) induced a rapid increase in the GH concentration. When atipamezole was used in combination with xylazine, it blunted the increase in the plasma GH concentration induced by the xylazine injection. The GH levels at 15-50 min after the simultaneous injection of xylazine and atipamezole were significantly (P<0.05) lower than the corresponding values in the animals given xylazine alone. The area under the GH response curve for 120 min after the simultaneous injection of xylazine and atipamezole was significantly (P<0.05) smaller than that for the xylazine alone. A series of five intravenous injections of 1 mg of SRIF at 10-min intervals also blunted xylazine-stimulated GH release. Atipamezole partially suppressed xylazine-induced hyperglycemia, but SRIF completely suppressed the hyperglycemia for the first 60 min after the xylazine injection and the suppression by SRIF was stronger than that by atipamezole. On the other hand, both atipamezole and SRIF failed to blunt xylazine-induced hypoinsulinemia. The present results suggest that xylazine stimulates GH release via the alpha(2)-adrenergic pathway in cattle, but the mechanism of xylazine-induced hyperglycemia remains to be determined [2].
Three studies were undertaken on farmed red and red x wapiti deer to evaluate xylazine and a xylazine/fentanyl citrate/azaperone combination for velvet antler removal. In the first experiment, 30 1-2 year-old red and 25% red x wapiti deer whose velvet was to be removed were given either 5% xylazine alone at 0.5 mg/kg body weight intramuscularly or the same dose rate of a commercially available mixture of 5% xylazine with the addition of 0.4 mg of fentanyl citrate and 3.2 mg of azaperone per mi. Physiological, behavioural and analgesic responses and reversal times after yohimbine or yohimbine and naloxone were monitored. There were no differences in heart rate, respiration rate, sedative or analgesic properties detected between xylazine or the xylazine/fentanyl citrate/azaperone combination. All deer became recumbent, but those given the xylazine/fentanyl citrate/azaperone combination became recumbent more rapidly than those given xylazine alone (9.4 and 12.5 minutes, respectively, p<0.05). The arousal pattern and timing of reversal of xylazine and xylazine/fentanyl citrate/azaperone using yohimbine and yohimbine and naloxone, respectively, were similar. The second experiment evaluated the reversal of the xylazine/fentanyl citrate/azaperone combination with either yohimbine or yohimbine and naloxone in 43 3-year-old red deer stags after velvet antler removal. There were no differences in arousal pattern or time to standing between reversal treatments. Sixteen 1-year-old red and 25% red x wapiti stags were used in the third experiment to evaluate clinically the analgesic properties of xylazine and xylazine/fentanyl citrate/azaperone combination during velvet removal without the application of a local anaesthetic agent. Withdrawal responses were observed in most deer after the xylazine/fentanyl citrate/azaperone combination at dosages containing 0.5, 0.7 and 0.75 mg of xylazine/kg and after xylazine alone at 0.7 mg/kg, indicating that insufficient analgesia was provided by the systemic agent for the surgical procedure of velvet antler removal. These studies, have shown that the knock-down effect of the xylazine/fentanyl citrate/azaperone combination was more rapid than that of xylazine alone, but that other physiological, behavioural and analgesic responses at doses used and evaluated by the methods used were similar. Reversal of both the xylazine and xylazine/fentanyl citrate/azaperone combination was similar when using either yohimbine alone for xylazine and the xylazine/fentanyl citrate/azaperone combination or yohimbine and naloxone for the xylazine/fentanyl citrate/azaperone combination. The evaluation of surgical analgesia for antler removal suggested that both xylazine alone and the xylazine/fentanyl citrate/azaperone combination provided insufficient analgesia and that local anaesthetic should be used in all cases [3].
References
[1] Xu L, Sui N. Method for chemical synthesis of 2,6-dimethylanilinothiazine[P]. Faming Zhuanli Shenqing, 109096222, 2018.
[2] KASUYA E, HODATE K, MATSUMOTO M, et al. Effects of Atipamezole, an α2-Adrenergic Antagonist, and Somatostatin on Xylazine-lnduced Growth Hormone Release in Calves[J]. Endocrine journal, 1996, 43(5): 551-556.
[3] Wilson P R, Beimans J, Stafford K J, et al. Xylazine and a xylazine/fentanyl citrate/azaperone combination in farmed deer I: Dose rate comparison[J]. New Zealand Veterin
See also
Lastest Price from Xylazine manufacturers

US $0.00/kg2025-03-18
- CAS:
- 7361-61-7
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10T

US $102.00/kg2025-01-16
- CAS:
- 7361-61-7
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000000 tons