Synthesis and application of s-tert-butyl sulfonamide
General description
Acid labile protecting groups are important in organic synthesis. The tert‐butyl group is commonly used for protection of a large variety of functional groups, e.g., acids, alcohols, phenols, and sulfonamides. Among them, s-tert-butyl sulfonamide is a chiral ligand used in pharmaceutical compositions[1-2]. P, n-sulfoxide imine ligands were synthesized by condensation of s-tert-butylsulfonamide with aldehydes and ketones, and they can be used for asymmetric hydrogenation of olefins under iridium catalysis[3].
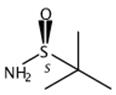
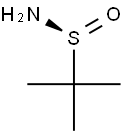
Fig. 1 The structure of S-tert-butyl sulfonamide.
Synthesis
Route1
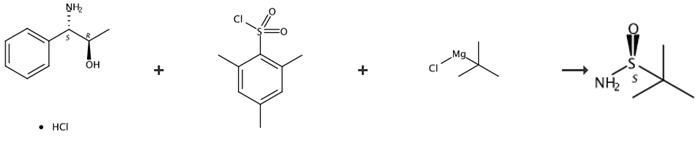
Fig. 2 The synthetic route of S-tert-butyl sulfonamide[4].
Step 1
Preparation of (1S, 2R)-N-(2-hydroxy-1-phenyl-propyl)-2,4,6-mesitylsulfonamide (49): neck round-bottomed flask equipped with an overhead stirrer and temperature probe, followed by methylene chloride (20 mL) and the mixture was cooled to 0°C and stirred for 15 minutes. 2-Mesitylenesulfonyl chloride (2.2 g, 10.1 mmol) was added in one portion and the slurry was mixed for 5 minutes. Triethylamine (2.7 g, 26.7 mmol) was added in 2 hours with stirring and the reaction was monitored by TLC for the disappearance of 2-mesitylenesulfonyl chloride. The reaction was quenched with saturated aqueous NaHCO3 (20 mL) and diluted with EtOAc (20 mL). The organic phase was washed with water (20 mL), 1.0 M HCl (10 mL), water (20 mL) and dried over Na2SO4. Evaporation of the organic solvent to dryness provided the title product in 95% yield (3.3 g). 1Η NMR (CDCl3): δ 1.02 (d, J = 6.35 Hz, 3H), 2.14 (d, J = 5.49 Hz, 1H), 2.21 (s, 3H), 2.49 (s, 6H), 4.07-4.11 (m, 1H), 4.15-4.18 (m, 1H), 5.70 (d, J = 7.21 Hz, 1H), 6.76 (s, 2H), 6.99-7.15 (m, 5H). 13C NMR (CDCl3): δ 19.5, 21.1, 23.1, 63.0, 70.4, 127.9, 128.3, 132.0, 134.3, 136.7, 139.0, 142.3. Anal: C18H23NO3S. Cal: C, 64.84; H, 6.95; N, 4.20, S, 9.62. Found: C, 65.19; H, 7.04; N, 4.18; S, 9.71.
Step 2
Preparation of (S)-t-butylsulfinamide ((S)8) from (S)-2-Methyl-2-propylsulfinic acid (1S, 2R)-1-methyl-2-phenyl-2-(2,4,6-mesitylsulfonylamino)-ethyl ester (51): A 50 mL three-necked round-bottomed flask equipped with a magnetic stir bar and an ammonia condenser was charged with 30 mL of liquid ammonia under Ar atmosphere. After the addition of a few crystals of Fe(NO3)3, lithium wire (0.05 g, 7.1 mmol) was added in a controlled manner and the internal temperature was kept around -45 °C. When all the lithium was added and a gray suspension was formed, the reaction mixture was cooled to -78 °C and a solution of (S)-2-methyl-2-ρroρylsulfinic acid (1S, 2R)-1-methyl-2-phenyl-2-(2,4,6-mesitylsulfonylamino)-ethyl ester (51) (0.45 g, 1.03 mmol) in THF (1 mL) was added slowly over a course of 20 minutes. Once the addition was complete, the mixture was warmed to -45 °C and stirred for 1 hour, followed by addition of NH4Cl (0.5 g). The cold bath was removed, and stirring continued until the mixture reached ambient temperature. The remaining volatile material was removed under reduced pressure. To the remaining residue was added 2 mL water and stirred. EtOAc (5 mL) was added to the mixture and stirred. After separation of the phases, the organic phase was washed with brine (2mL x 2). After removal of the organic solvent, the residue was purified by chromatography eluted with EtOAc to afforded (S)-t-butylsulfinamide (0.125 g, 99%) with 99% ee. (HPLC, Chiralpak AS column, 90:10 hexane/ethanol; 1.2 mL/min, 222 nm; (R)-TBSA rt = 6.6 min; (S)-TBSA, rt = 9.4 min). 1Η NMR (CDCl3): 5 1.18 (s, 9H), 3.82 (br, s, 2H). 13C NMR(CDCl3): δ 22.1, 55.3.
Route 2
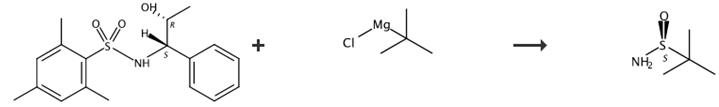
Fig. 3 The synthetic route of S-tert-butyl sulfonamide[5].
Step 1
(S)-2-Methyl-2-propylsulfinic acid (1S, 2R)-1-methyl-2-phenyl-2-(2,4,6-mesitylsulfonylamino)-ethyl ester (51): In a 50 mL two-necked, round-bottomed flask equipped with a magnetic stir bar, rubber septum, and argon inlet was placed (2S, 4S, 5R)-5-methyl-4-phenyl-3-(2,4,6-mesitylsulfonyl)-[1,2,3]oxathiazolidine 2-oxide (50) (0.58 g, 1.53 mmol) dissolved in THF (1.0 mL) and the mixture was cooled to -78 °C. A solution of t-butyl magnesium chloride (3.1 mL, 1.0 M) in THF was added dropwise via syringe for 30 minutes with stirring. After 1-2 hours, as monitored by TLC for the disappearance of the starting material, the reaction was quenched with saturated aqueous NaHCO3 (5 mL), and diluted with EtOAc (5 mL). The aqueous phase was extracted with EtOAc (4 mL) and the combined organic phases were washed with brine (5 mL), dried with (Na2SO4 and concentrated to afford a crystalline product (0.65 g. 97%) with >99% de (minor diasteriomer not detected). 1Η NMR (CDCl3): δ 1.080 (d, J = 6.47 Hz, 3H), 1.1749 (s, 9H), 2.163 (s, 3H), 2.485 (s, 6H), 4.394 (dd, J1 = 8.98 Hz, J2 = 2.32 Hz, 1H), 4.675 (dq, J1 = 2.32 Hz, J2 = 6.53 Hz, 1H), 6.61-6.67 (m, 2H), 6.96-7.09 (m, 5H). 13C NMR (CDCl3): δ 19.10, 20.90, 21.80, 22.98, 57.85, 61.10, 81.86, 127.58, 128.80, 131.61, 134.98, 135.10, 138.50, 141.55.
Step 2
Preparation of (S)-t-butylsulfinamide ((S)8) from (S)-2-Methyl-2-propylsulfinic acid (1S, 2R)-1-methyl-2-phenyl-2-(2,4,6-mesitylsulfonylamino)-ethyl ester (51): A 50 mL three-necked round-bottomed flask equipped with a magnetic stir bar and an ammonia condenser was charged with 30 mL of liquid ammonia under Ar atmosphere. After the addition of a few crystals of Fe(NO3)3, lithium wire (0.05 g, 7.1 mmol) was added in a controlled manner and the internal temperature was kept around -45 °C. When all the lithium was added and a gray suspension was formed, the reaction mixture was cooled to -78 °C and a solution of (S)-2-methyl-2-ρroρylsulfinic acid (1S, 2R)-1-methyl-2-phenyl-2-(2,4,6-mesitylsulfonylamino)-ethyl ester (51) (0.45 g, 1.03 mmol) in THF (1 mL) was added slowly over a course of 20 minutes. Once the addition was complete, the mixture was warmed to -45 °C and stirred for 1 hour, followed by addition of NH4Cl (0.5 g). The cold bath was removed, and stirring continued until the mixture reached ambient temperature. The remaining volatile material was removed under reduced pressure. To the remaining residue was added 2 mL water and stirred. EtOAc (5 mL) was added to the mixture and stirred. After separation of the phases, the organic phase was washed with brine (2mL x 2). After removal of the organic solvent, the residue was purified by chromatography eluted with EtOAc to afforded (S)-t-butylsulfinamide (0.125 g, 99%) with 99% ee. (HPLC, Chiralpak AS column, 90:10 hexane/ethanol; 1.2 mL/min, 222 nm; (R)-TBSA rt = 6.6 min; (S)-TBSA, rt = 9.4 min). 1Η NMR (CDCl3): 5 1.18 (s, 9H), 3.82 (br, s, 2H). 13C NMR(CDCl3): δ 22.1, 55.3.
Application
S-tert-butyl sulfonamide is an ammonia equivalent for the palladium-catalyzed amination of aryl bromides and aryl chlorides[6]. Using these amine derivatives, it has been observed that indoles and anilines with sensitive functional groups can be readily prepared. This surrogate has also been used for the synthesis of indoles from 2-halophenols using palladium catalyzed cross coupling reaction as the key step[7-8]. The tert-butyl sulfinyl group can be selectively cleaved without affecting other functional groups establishes tert-butyl sulfinamide as an excellent ammonia surrogate for aryl amination reaction. Moreover, utilizing this surrogate, a general and efficient approach for the synthesis of indoles from o-halophenol is presented[9].
Hazard statement
Swallowing s-tert-butyl sulfonamide is harmful. It will also cause strong irritation to the skin, and even cause serious eye irritation. In addition, it may cause respiratory tract irritation. Additionally, s-tert-butyl sulfonamide can stimulate the mucous membrane of eyes and respiratory tract[10]. Due to its low volatility, its stimulating effect is insufficient to cause serious harm.
Reference
[1] M. Cooper, D. Miller, A. MacLeod, S. Thom, S. St-Gallay, J. Shannon, T. Alanine, S. Onions, I. Strutt, Preparation of sulfonylureas and sulfonylthioureas as NLRP3 inhibitors, Inflazome Limited, Ire. . 2019, p. 370pp.
[2] R. De Francesco, L. Donnici, L. Guidotti, M. Iannacone, R. Di Fabio, V. Summa, L.I. Bencheva, M. De Matteo, L. Ferrante, A. Prandi, P. Randazzo, Preparation of arylaminocarbonyl bicyclic sulfonamides as inhibitors of hepatitis B virus, IRMB S.p.A., Italy; Promidis S.r.l.; Ospedale San Raffaele S.r.l.; Istituto Nazionale di Genetica Molecolare-INGM . 2020, p. 176pp.
[3] R. De Francesco, L. Donnici, L. Guidotti, M. Iannacone, R. Di Fabio, V. Summa, L.I. Bencheva, M. De Matteo, L. Ferrante, A. Prandi, P. Randazzo, Preparation of arylaminocarbonyl bicyclic sulfonamides as inhibitors of hepatitis B virus, Ospedale San Raffaele S.r.l., Italy; IRMB S.p.A.; Promidis S.r.l.; Istituto Nazionale di Genetica Molecolare - INGM . 2020, p. 238pp.
[4] R. De Francesco, A. Prandi, P. Randazzo, L. Donnici, L. Guidotti, M. Iannacone, R. Di Fabio, V. Summa, L.I. Bencheva, M. De Matteo, L. Ferrante, Preparation of arylaminocarbonyl bicyclic sulfonamides as inhibitors of hepatitis B virus, Ospedale San Raffaele S.r.l., Italy; IRBM S.p.A.; Promidis S.r.l.; Istituto Nazionale di Genetica Molecolare - INGM . 2020, p. 108pp.
[5] T. Focken, J.-C. Andrez, K.N. Burford, C.M. Dehnhardt, M.E. Grimwood, Q. Jia, V.A. Lofstrand, S.S. Wesolowski, M.S. Wilson, Preparation of heteroaryl-substituted sulfonamide compounds and their use as sodium channel inhibitors, Xenon Pharmaceuticals Inc., Can. . 2020, p. 279pp.
[6] T. Focken, J.-C. Andrez, K.N. Burford, C.M. Dehnhardt, M.E. Grimwood, Q. Jia, V.A. Lofstrand, M.S. Wilson, A.Y. Zenova, S.S. Wesolowski, S. Sun, Preparation of heteroaryl-substituted sulfonamide compounds and their use as sodium channel inhibitors, Xenon Pharmaceuticals Inc., Can. . 2020, p. 205pp.
[7] K. Kreutter, L. Whitehead, D.L. Romero, S.K. Albanese, H. Koldsoe, A. Verras, Preparation of fused heterocyclic sulfonamides IRE1α modulators and uses thereof, Nimbus Iaso, Inc., USA . 2021, p. 172pp.
[8] M. Lin, J. Luo, Y. Xie, G. Du, Z. Cai, B. Dai, L. He, Organocatalytic silicon-free SuFEx reactions for modular synthesis of sulfonates and sulfonamides, ChemRxiv (2021) 1-21.
[9] D. Sarkar, E.T. Olejniczak, J. Phan, J.A. Coker, J. Sai, A. Arnold, Y. Beesetty, A.G. Waterson, S.W. Fesik, Discovery of Sulfonamide-Derived Agonists of SOS1-Mediated Nucleotide Exchange on RAS Using Fragment-Based Methods, J. Med. Chem. 63(15) (2020) 8325-8337.
[10] K. Schroder, A. Robertson, M. Cooper, R. Coll, L. O'Neill, Sulfonylurea related compound for treating autoimmune disorder, The University of Queensland, Australia; The Provost, Fellows, Foundation Scholars, and the Other Members of Board . 2019, p. 528pp.
See also
Lastest Price from (S)-(-)-2-Methyl-2-propanesulfinamide manufacturers
US $0.00/kg2025-08-29
- CAS:
- 343338-28-3
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 20tons
US $0.00/kg2025-07-18
- CAS:
- 343338-28-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS