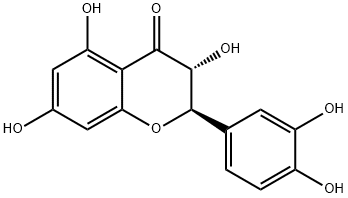
Synthesis and application of Taxifolin
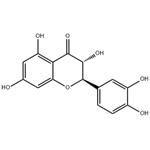
Taxifolin, a very important dihydroflavonol compound with the formula of C15H12O7, is an ordorless, colorless acicular crystal or in pale yellow powder. Due to the existence of two chiral carbon atoms in the molecule, taxifolin has four chiral isomers theoretically, and the CAS numbers are 11003-33-9, 153666-25-2, 114716-89-6, and 480-18-2, respectively. It is easily soluble in polar solvents including ethanol, acetic acid and hot water, slightly soluble in cold water and insoluble in non-polar solvents like benzene [1]. The melting point is 240-242 ℃ in hot water and 208-210 ℃ in 50% ethanol.

Extration
Taxifolin is commonly found in various plants like larches, especially in the heartwood of Douglas fir [2]. It can also be found in grapes, citrus fruits, onions, green tea, olive oil, wine and so on. In larches, taxifolin exists in a monomer form, which can be extracted using boiling water or suitable solvents like acetone and ethanol [3]. Some researchers also utilize sonication, microwave or biological enzymes to accelerate the extraction process [4, 5]. In some other plants like Engelhardia, taxifolin is in the form of its glycoside derivative, therefore the hydrolysis procedure is needed to acquire taxifolin.
Synthesis
The extraction method is limited by the rather low content of taxifolin in plants and the great consumption of the solvents. Therefore, the synthesis of taxifolin becomes increasingly important. Several reactions can be used to synthesize taxifolin, including Algar-Flynn-Olyamada (AFO) reaction, Mitsunobu reaction and semisynthetic method.
(1) AFO reaction
Taxifolin belongs to dihydroflavanols in flavonoids. AFO reaction is a common way to prepare 3-hydroxyl flavanols, and dihydroflavanol is an intermediate of this reaction. By controlling the reaction conditions appropriately, Taxifolin can be obtained by keeping the reactants in the dihydroflavonol stage. For example, the reaction routes of Sun Shuxiang et al are as follows in Figure 1 [5].
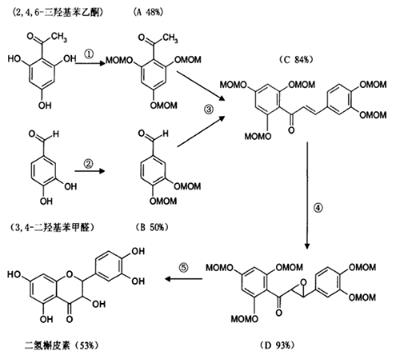
Figure 1. The synthesis of taxifolin by AFO reaction
(2) Mitsunobu reaction
Invented by Japanese chemist Oyo Mitsunobu et al in 1967, Mitsunobu reaction is a commonly used reaction in modern organic synthesis. Using triphenyl as the condensation agent, the esterification reaction between carboxylic acid and alcohol can happen. The reaction requires mild conditions and the product has optical activity. The synthesis of taxifolin using this reaction in the work of Sang-Sup Jew et al is as follows in Figure 2 [6].

Figure 2. The synthesis of taxifolin by Mitsunobu reaction
(3) Semisynthetic technique
Semisynthetic technique uses natural products derived from animals, plants, or microorganisms as starting materials to synthesis final products, and is commonly used in industry because of its high yield. Souhila Ghidouche et al. described the convenient preparation of dihydroquercetin from common extract catechins by semi-synthesis in 2007 [7]. The reaction route is as follows in Figure 3.

Figure 3. The synthesis of taxifolin by semisynthetic technique
Application
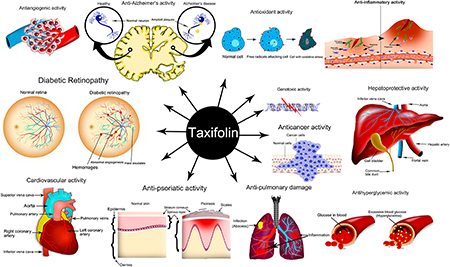
Taxifolin is an antioxidant flavonoid and has the P-vitamin activity, making it potential in clinical applications. It presents a number of important and useful pharmacological properties [8], such as:
(1) Termination of free radicals and peroxidation prevention;
(2) Inactivation of cytotoxic substances;
(3) Antidiabetic activity by reducing sugar, uric acid and creatinine in human blood;
(4) Reduction of lipoprotein content in liver and blood plasma;
(5) Immunoregulatory (antiallergic) and anti-inflammatory activity;
(6) Decreasing the accumulation of b-amyloid and preventing memory deficits;
(7) Promoting osteogenic differentiation in human bone marrow mesenchymal stem cells.
(8) Normalizing effect on cell fermentative systems;
(9) Pain-relieving and anti-edema action;
(10) Low mutagenic activitytoxicity;
(11) Antitumour, antiviral and antiradiation activity,
(12) Antimutagene and activity.
Apart from the abundant clinical applications, taxifolin can also be used as an antioxidant additive in food for its nontoxicity and sweet flavor. Due to its colorless nature in solvents, taxifolin is ideal for white or transparent food like vegetable oils.
Besides, it can be added to some cosmetics as the main functional ingredient, and also works as an oxidizing stabilizer in rocket fuel, hydrocarbon fuel, commercial grease and so on.
Safety
Tilll now, no toxic effects has been found when taxifolin was applied to animals in large doses, and no teratogenic, allergenic or mutagenic effects on fetuses have been found.
References
[1] Dong L, Cao H, Zhang X, Wang H. Research progress on Dihydroquercetin [J].
Current Biotechnology, 2020, 10 (3), 226-233.
[2] https://www.merriam-webster.com/dictionary/taxifolin
[3] Liu Z Z, Wei M X, Cui G Q, et al. Optimization of arabinogalactan and taxifolin extraction process from Dahurian larch (Larix gmelinii) and evaluation of the effects on activities of -amylase, -glycosidase, and pancreatic lipase in vitro [J]. Journal of Food Biochemistry, 2018, 42 (5), 12607.
[4] Liu Z Z, Jia J, Chen F L, et al. Development of an ionic liquid-based microwave-assisted method for the extraction and determination of Taxifolin in different parts of larix gmelinii [J]. Molecules, 2014, 19 (12), 19471-19490.
[5] El-Adawi H, Abdel-Fattah Y, Abd El-Wahab A. Application of numerical modeling for optimization of selective hot water extraction of taxifolin from ‘milk thistle’ seeds [J]. African Journal of Biotechnology, 2011, 10 (48), 9804-9811.
[6] Jew S, Kim H, Bae S, et al. Enantioselective synthetic method for 3-hydroxyflavanones: an approach to (2R,3R)- 3′,4′-O-dimethyltaxifolin [J]. Tetrahedron Letters, 2000, 41 (41), 7925-7928.
[7] Es-Safi N E, Ghidouche S, Ducrot P H. Flavonoids: Hemisynthesis, reactivity, characterization and free radical scavenging activity [J]. Molecules, 2007, 12 (9), 2228-2258.
[8] Koroteev A M, Kukhareva T S , Koroteev M P , et al. Synthesis and Properties of New Dihydroquercetin Derivatives[J]. Journal of Pharmacy and Pharmacology, 2015, 3 (2): 43-57.
You may like
Related articles And Qustion
See also
Lastest Price from Taxifolin manufacturers
US $0.00-0.00/kg2025-09-08
- CAS:
- 480-18-2
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1
US $0.00-0.00/kg2025-04-27
- CAS:
- 480-18-2
- Min. Order:
- 0.10000000149011612kg
- Purity:
- 99%
- Supply Ability:
- 100kg