Uses and Preparation of (S)-1-Boc-3-hydroxypiperidine
General description
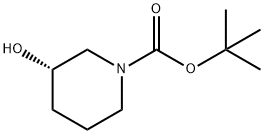
The (S)-1-Boc-3-hydroxypiperidine, with the CAS No: 143900-44-1, is also known as (S)-N-Boc-3-hydroxypiperidine, (S)-tert-Butyl 3-hydroxypiperidine-1-carboxylate, (S)-1-N-Boc-3-Hydroxy-piperidine. This chemical’s molecular formula is C10H19NO3 and molecular weight is 201.26 (Figure 1). As a piperidine derivative, (S)-1-tert-butoxycarbonyl-3-hydroxypiperidine (1) is an important intermediate with a chiral structure, which has antibacterial, antitumor, and treatment of Alzheimer's disease. It is also one of the important drugs for the treatment of viral infections (including AIDS) and diabetes [1].
Figure 1 The molecular formula of (S)-1-Boc-3-hydroxypiperidine
USE
Many natural products and active pharmaceutical ingredients share a common piperidine core, and the introduction of a chiral hydroxyl group on the C3-position of the piperidine ring may alter the bioactivity of the molecule. (S)-1-Boc-3-hydroxypiperidine ((S)-NBHP) is a key chiral intermediate in the synthesis of ibrutinib as the inhibitor of Bruton’s tyrosine kinase, which is used in the treatment of B-cell malignancies, has been approved by the US Food and Drug Administration in 2013 for the treatment of mantle cell lymphoma, in 2014 for the treatment of chronic lymphocytic leukemia, and in January 2015 for the treatment of lymphoplasmacytic lymphoma[2,3]. Piperidine derivatives have many pharmacological activities and are characteristic substructures of many bioactive natural products, drugs, or drug candidates. With the expansion of its indications such as chronic lymphocytic leukaemia and lymphoplasmacytic lymphoma, its key intermediate (S)-1-Boc-3-hydroxypiperidine is considered to be a highly useful synthetic synthon in the pharmaceutical industry.
Preparation
The synthetic methods of (S)-1-Boc-3-hydroxypiperidine reported in the literature are mainly divided into chemical resolution method and biotransformation method.
1)Chemical resolution method. This method usually uses 3-hydroxypiperidine to be separated by tartaric acid derivative or camphorsulfonic acid salt, and then protected by tert-butoxycarbonyl to obtain (S)-1-tert-butoxycarbonyl-3-hydroxypiperidine (Scheme 1) . However, such methods have the disadvantages of low yield, many unit operations, and high cost.
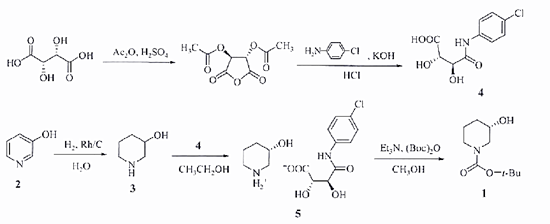
Scheme 1. Synthesis of (S)-1-Boc-3-hydroxypiperidine by Chemical Resolution
In addition, methods such as classical diastereomeric decomposition, asymmetric synthesis and asymmetric reduction are also included. For example, an asymmetric synthesis approach to generate (S)-1-tert-butoxycarbonyl-3-hydroxypiperidine from achiral 4-methyl phenacyl bromide via a 13-step conversion with a 35% yield was reported [4]. While the diastereomeric resolution was reported to be the main chemical process to produce it by using 3-hydroxypiperidine as starting material. These methods also suffered from low yields or lengthy synthesis.
2)Biotransformation method. Using 4 as a substrate, a highly efficient and green enzyme-catalyzed synthesis of (S)-1-Boc-3-hydroxypiperidine was carried out[5]. 2 is protected by tert-butoxycarbonyl to give 1-tert-butoxycarbonyl-3-hydroxypiperidine, 3 is oxidized by sodium hypochlorite-TEMPO to give 4, and 4 is efficiently chiral and selectively converted to (S)-1-Boc-3-hydroxypiperidine by ketoreductase (Scheme 2).
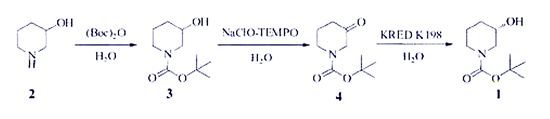
Scheme 2. Synthesis of (S)-1-Boc-3-hydroxypiperidine by Biotransformation
Asymmetric reduction of N-Boc-piperidin-3-one (NBPO) by reductases is a practical way to produce (S)-1-Boc-3-hydroxypiperidine, since it can be carried out under mild reaction conditions, and results in high yield and remarkable enantioselectivity. Alternatively, the carbonyl-reductase-catalyzed asymmetric reduction of N-Boc-3-piperidone (NBPO) has gained increasing focus due to its mild reaction conditions, high yield, and remarkable enantioselectivity.
Precautions for safety handling [6]
Classification according to Regulation (EC) No 1272/2008
Skin irritation (Category 2), H315 Eye irritation (Category 2), H319 Specific target organ toxicity - single exposure (Category 3), Respiratory system, H335 For the full text of the H-Statements mentioned in this Section, see Section 16.
GHS Hazard Statements
Causes skin irritation [Warning Skin corrosion/irritation]; Causes serious eye irritation [Warning Serious eye damage/eye irritation]; May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation].
Handling and storage
Avoid breathing dust/ fume/ gas/ mist/ vapors/ spray. Wash skin thoroughly after handling. Use only outdoors or in a well-ventilated area. Wear protective gloves/ eye protection/ face protection. IF ON SKIN: Wash with plenty of water. IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing[7]. Keep away from sources of ignition - No smoking. Store in cool place. Keep container tightly closed in a dry and well-ventilated place. The storage place must be locked.
References
- Reddy MS,Narender M,Rao KR.Anew asymmetric synthetic route to substituted piperidines[J].Tetrahedron,2007,63(2):331-336.
- Pan ZY,Scheerens H,Li SJ,et al.Discovery of selective irreversible inhibitors for Bruton's tyrosine kinase[J].Chem Med Chem,2007,2(1):58-61.
- Xu, GP (Xu, Guang-Peng)1,2,3;Wang, HB (Wang, Hai-Bo)1,2,3;Wu, ZL (Wu, Zhong-Liu)1,2.Efficient bioreductive production of (S)-N-Boc-3-hydroxypiperidine using ketoreductase ChKRED03[J].Process Biochemistry.2016,Vol.51(No.7):881-885.
- M. Somi Reddy; M. Narender; K. Rama Rao.A new asymmetric synthetic route to substituted piperidines.[J].Tetrahedron.2007,Vol.63(No.2):331-336.
- ZHU Wei, WANG Bo, WU Hui, LI Binghao. A Chemo-enzyme Method to Synthesis of(S)-t-Butyl 3-Hydroxypiperidine-1-carboxylate[J].Chinese Journal of Pharmaceuticals,2015,46(04):349-350.
- MSDS sigmaaldrich.com.
- National Center for Biotechnology Information. "PubChem Compound Summary for CID 1514399, (S)-1-Boc-3-hydroxypiperidine" PubChem.
See also
Lastest Price from (S)-1-Boc-3-hydroxypiperidine manufacturers

US $0.00/kg2025-04-21
- CAS:
- 143900-44-1
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg

US $50.00/kg2025-04-21
- CAS:
- 143900-44-1
- Min. Order:
- 1kg
- Purity:
- 99
- Supply Ability:
- 5000