Description, Synthesis and Usage of Phenylhydrazine
General description
Phenylhydrazine is a white monoclinic prismatic crystal or oily liquid, which has an aromatic smell and turns yellow in the air. Notably it is poisonous. Its physical and chemical properties include melting point of 19.5 ℃ (melting point of phenylhydrazine hydrate 24 ℃), boiling point of 243.5 ℃, relative density of 1.0978, refractive index of 1.60813, flash point of 69 ℃, weak basicity [ka (15 ℃) 1.62 × 10-9][1]。 It is miscible with ethanol, ether and benzene, and slightly soluble in water and petroleum ether. Phenylhydrazine can be obtained by diazotization and NaHSO3 reduction. It is an important intermediate in the dye, pharmaceutical and pesticide industries.
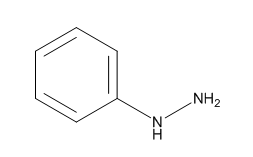
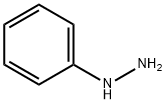
Fig. 1 The structure of phenylhydrazine.
Physical property data
Phenylhydrazine shows light yellow crystal or oily liquid, with pungent smell. Its melting point (°C) is 19.4, the boiling point (°C) is 243.5 (decomposition), the relative density (water =1) is 1.10 [2], the relative vapor density (air =1) is 3.7, the Saturated vapor pressure (kPa) is 1.33 (115℃), the octanol/water partition coefficient is 1.9, the flash point (°C) is 89 (CC) [3], the ignition temperature (°C) is 615, the lower limit of explosion (%) is 1.3, and the refractive index is 1.6084. It is insoluble in cold water, slightly soluble in hot water, mixed soluble in ethanol, ether, benzene and most organic solvents [4].
Synthesis
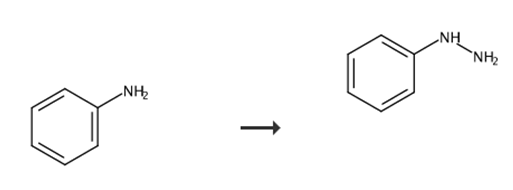
Fig. 2 Synthetic route of phenylhydrazine.
Add concentrated hydrochloric acid (10 mL) to a solution of the aniline (10.0 mmol) in acetic acid (5.0 mL) at room temperature.
After cooling to 0 °C, add a solution of NaNO2 (830 mg, 12.0 mmol) in water (3.0 mL) dropwise to the mixture over a period of 20 minutes.
Continue stirring at 0 °C for further 30 minutes.
Add a precooled solution of tin(II) chloride dihydrate (5.00 g, 22.0 mmol) in concentrated hydrochloric acid (10 mL) dropwise to the mixture over a period of 10 minutes.
After 1 hour of stirring at 0 °C, collect the precipitate by filtration.
Wash the precipitate with water.
Dry the precipitate in vacuo [6].
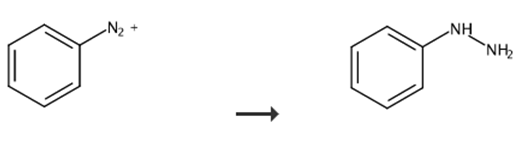
Fig. 3 Synthetic route of phenylhydrazine.
Step 1
Apart from the above-mentioned container, a reaction vessel with 4 mouth flask of 2L capacity equipped with a stirrer, a thermometer, a Dimroth condenser was prepared. Within this reaction vessel, sodium sulfite 97.4 g (97% purity) was dissolved in water 350g, 95% sulfuric acid was added, and the pH was adjusted to 7.2. This solution was cooled to 10 °C, and the diazonium salt solution prepared previously was added quickly. After raising the temperature to 20 °C over 30 minutes, this obtained mixture was heated to 65 °C and by stirring at the same temperature for 2 hours, the diazonium salt was reduced. A mixture containing phenyl hydrazine sulfonate was obtained.
Step 2
After adding 200 g of toluene to the obtained mixture, it was stirred for 15 minutes at 65 °C, the aqueous layer was washed and after removing the unreacted raw material and the reaction by-product, liquid was separated on standing. The separated water layer was poured into the reaction vessel with 4 mouth flask of 3L capacity equipped with stirrer, thermometer, and Dimroth condenser, and then 35% hydrochloric acid 313g was added dropwise over 20 minutes at 20 °C, it was stirred at 75 °C for 3 hours, and phenyl hydrazine sulfonate previously obtained was hydrolyzed, a mixture containing the hydrochloride salt of phenylhydrazine was obtained. This mixture was cooled to 10 °C, 50% sodium hydroxide aqueous solution 300g was added slowly, phenyl hydrazine was precipitated, subsequently, the aqueous layer was filtered, removed and recovered. Crude product of phenyl hydrazine was obtained. The obtained phenyl hydrazine crude product was 34g. (90% purity) [7].
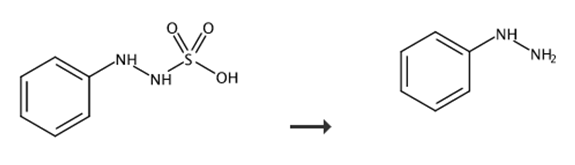
Fig. 4 Synthetic route of phenylhydrazine.
After adding 200 g of toluene to the obtained mixture, it was stirred for 15 minutes at 65 °C, the aqueous layer was washed and after removing the unreacted raw material and the reaction by-product, liquid was separated on standing. The separated water layer was poured into the reaction vessel with 4 mouth flask of 3L capacity equipped with stirrer, thermometer, and Dimroth condenser, and then 35% hydrochloric acid 313g was added dropwise over 20 minutes at 20 °C, it was stirred at 75 °C for 3 hours, and phenyl hydrazine sulfonate previously obtained was hydrolyzed, a mixture containing the hydrochloride salt of phenylhydrazine was obtained. This mixture was cooled to 10 °C, 50% sodium hydroxide aqueous solution 300g was added slowly, phenyl hydrazine was precipitated, subsequently, the aqueous layer was filtered, removed and recovered. Crude product of phenyl hydrazine was obtained. The obtained phenyl hydrazine crude product was 34g. (90% purity) [8].
Phenylhydrazine derivative
Hydrazine derivative phenylhydrazine, also known as biaminobenzene, was first synthesized by German organic chemist Hermann Emile Fischer in 1875 and was the first synthetic hydrazine derivative. At room temperature, it is a light yellow crystal or oily liquid, and at low temperature, it is monoclinic prism crystal, which is easy to be oxidized in air and is dark brown or deep red [9]. One of the hydrazine derivatives, often abbreviated PhNHNH2. The commonly used preparation method is through aniline and sodium nitrite under the action of hydrochloric acid to generate diazonium salt, and then Phenylhydrazine hydrochloride is formed by acid chromatography, and phenylhydrazine is obtained by neutralization. It is also an important reagent for the identification of carbonyls, aldehydes, ketones and sugars. It reacts with benzaldehyde to form phenylhydrazone, and aldehydes and ketones are identified by the hydrazone formed by phenylhydrazine or 2, 4-dinitrophenylhydrazine. Fischer indole synthesis occurs with aldehydes and ketones (discovered by Hermann Emile Fischer in 1883) [10]. The reaction is to remove a molecule of ammonia by heating and rearranging phenylhydrazine with aldehydes and ketones under the catalysis of acid to obtain 2- or 3-substituted indole. Indole cyclic compounds are eventually obtained.
Storage methods
Phenylhydrazine is stored in a cool, well-ventilated warehouse away from ignition and heat sources. Packing should be sealed and out of contact with air. It should be stored separately from oxidizer and edible chemicals, and must not be mixed. The appropriate variety and quantity of fire equipment is properly configured [11]. The storage area shall be equipped with leakage emergency treatment equipment and suitable storage materials.
Main purpose
Phenylhydrazine is used in the production of dyes, drugs, developer, etc. It is also an important reagent for identifying carbonyls, aldehydes, ketones, and sugars. In addition, it can also be used as a characteristic derivative reagent for the spectrophotometric determination of phosphorus and selenium reducing agent aldehydes and ketones. In addition, it is also used in organic synthesis, as analytical reagents and the manufacture of dyes and drugs [12].
Toxicology data
Acute toxicity: LD50:188mg/kg (rat oral); 175mg/kg (mouse oral); 80mg/kg (rabbit mouth); 90mg/kg (percutaneous rabbit)
Gene transformation and mitotic recombination: Saccharomyces cerevisiae 25mg/L. DNA damage: Mice were given 350μmol/kg intraperitoneally [13].
The minimum toxic dose (TDLo) in the abdominal cavity of rats was 30mg/kg (17-19d of gestation), which affected the behavior of newborn rats.
Characteristics
Flammable in case of open fire and high heat. May react with a strong oxidant. Heat decomposition gives off toxic nitrogen oxide smoke.
It turns reddish brown in the air and is toxic, causing hemolysis of red blood cells [14].
Stability: Stability
Forbidden compounds: strong oxidants
Avoid contact conditions: exposure to heat, light and air
Polymerization harm: no polymerization
Ecological data
Ecotoxicity:
LC50:0.16-0.26 mg/L (96h) (zebrafish); 21.4mg/L (24h), 15.7mg/L (48h) (Medaka, static)
EC50: < 1200μg/L (24h) (Daphnia, static)
Biodegradability: Static Zahn-Wellens test, degradation of 85% in 9~13d [15].
Non-biodegradability: When the concentration of hydroxyl radical is 5.00×105 /cm3 in air, the degradation half-life is 9h (theoretical) [16].
References
[1] M. Alam, A.M. Asiri, M.M. Rahman, Fabrication of phenylhydrazine sensor with V2O5 doped ZnO nanocomposites, Materials Chemistry and Physics 243 (2020) 122658.
[2] J. Berger, Phenylhydrazine haematotoxicity, J Appl Biomed 5(3) (2007) 125-30.
[3] D.Y. Curtin, E. Tristram, The Reaction of Phenylhydrazine with α-Haloacetophenones1, Journal of the American Chemical Society 72(11) (1950) 5238-5242.
[4] A. Diallo, M. Gbeassor, A. Vovor, K. Eklu-Gadegbeku, K. Aklikokou, A. Agbonon, A.A. Abena, C. de Souza, K. Akpagana, Effect of Tectona grandis on phenylhydrazine-induced anaemia in rats, Fitoterapia 79(5) (2008) 332-336.
[5] M. Ferrali, C. Signorini, L. Ciccoli, M. Comporti, Iron release and membrane damage in erythrocytes exposed to oxidizing agents, phenylhydrazine, divicine and isouramil, Biochemical Journal 285(1) (1992) 295-301.
[6] H. Hara, M. Ogawa, Erythropoietic precursors in mice with phenylhydrazine‐induced anemia, American journal of hematology 1(4) (1976) 453-458.
[7] S. Jain, D. Subrahmanyam, On the mechanism of phenylhydrazine-induced hemolytic anemia, Biochemical and Biophysical Research Communications 82(4) (1978) 1320-1324.
[8] X.-B. Li, Q.-Y. Wu, C.-Z. Wang, J.-H. Lan, M. Zhang, Z.-F. Chai, W.-Q. Shi, Theoretical Insights into the reduction mechanism of Np (VI) with phenylhydrazine, The Journal of Physical Chemistry A 125(28) (2021) 6180-6188.
[9] S. Luangaram, U. Kukongviriyapan, P. Pakdeechote, V. Kukongviriyapan, P. Pannangpetch, Protective effects of quercetin against phenylhydrazine-induced vascular dysfunction and oxidative stress in rats, Food and chemical toxicology 45(3) (2007) 448-455.
[10] H.P. Misra, I. Fridovich, The oxidation of phenylhydrazine: superoxide and mechanism, Biochemistry 15(3) (1976) 681-687.
[11] A. Nezamzadeh-Ejhieh, F. Khodabakhshi-Chermahini, Incorporated ZnO onto nano clinoptilolite particles as the active centers in the photodegradation of phenylhydrazine, Journal of Industrial and Engineering Chemistry 20(2) (2014) 695-704.
[12] K. Pandey, A.K. Meena, A. Jain, R. Singh, Molecular mechanism of phenylhydrazine induced haematotoxicity: a review, Ame J Phytomed Clin Therapeut 2(3) (2014) 390-394.
[13] D. Ringe, G.A. Petsko, D.E. Kerr, P.R. Ortiz de Montellano, Reaction of myoglobin with phenylhydrazine: a molecular doorstop, Biochemistry 23(1) (1984) 2-4.
[14] A.E. Rosamilia, F. Arico, P. Tundo, Reaction of the ambident electrophile dimethyl carbonate with the ambident nucleophile phenylhydrazine, The Journal of Organic Chemistry 73(4) (2008) 1559-1562.
[15] M.D. Shetlar, H. Hill, Reactions of hemoglobin with phenylhydrazine: a review of selected aspects, Environmental health perspectives 64 (1985) 265-281.
[16] P. Shukla, N.K. Yadav, P. Singh, F. Bansode, R. Singh, Phenylhydrazine induced toxicity: a review on its haematotoxicity, Int J Basic Appl Med Sci 2(2) (2012) 86-91.
See also
Lastest Price from Phenylhydrazine manufacturers

US $1.00/KG2025-06-09
- CAS:
- 100-63-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 50 ton

US $25.00/ASSAYS2025-03-10
- CAS:
- 100-63-0
- Min. Order:
- 100ASSAYS
- Purity:
- 99.5%
- Supply Ability:
- 100 mt