Synthesis and Application of Ropivacaine
General description
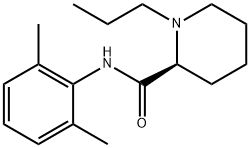
The chemical name of ropivacaine is S-(-)-1-propyl-N-(2,6-xylenyl)-2-piperidine formamide. It is the first pure levo long-acting amide local anesthetic, which has both anesthetic and analgesic effects. Large doses can produce surgical anesthesia, while small doses can produce sensory block (analgesia) with limited non progressive motor block.
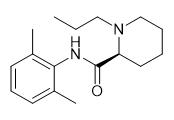
Fig. 1 The structure of Ropivacaine.
Synthetic routes
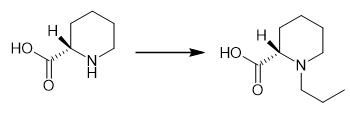
Fig. 2 The synthetic step 1 of Ropivacaine.
(S) -piperidine 2-formic acid (100.00 g,0.77 mol), propionic aldehyde (104.32 g, 2.32 mol) and methylene chloride (800 mL) were added to the 2000 mL reaction flask, and the air in the reaction flask was replaced by nitrogen. Then sodium cyanoborohydride (111.90g, 2.30mol) was added and the reaction was carried out at 35℃ for 10 hours. After the reaction, the organic phase was quenched with saturated sodium bicarbonate aqueous solution (500mL) to separate the organic phase. The aqueous phase was extracted with methylene chloride (500mL*2), and the organic phase was combined and dried with anhydrous magnesium sulfate. At 40℃ ~ 45℃, 125 g of intermediate 1 was concentrated under reduced pressure with a yield of 94.3% [1].
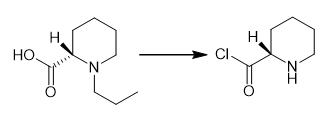
Fig. 3 The synthetic step 2 of Ropivacaine.
Intermediates 1 (100.00 g, 0.58 mol), methylene chloride (600 mL) and N, N-dimethylformamide (2 mL) were added to the 2000 mL reaction flask. The temperature was cooled to 5℃ in an ice water bath, and sulfoxide chloride (76.42g, 0.64mol) was added slowly to control the internal temperature ≤10℃. After addition, the ice water bath was removed, and the mixture was naturally restored to room temperature, and then heated to the internal temperature of 40℃ for reflux reaction for 4 hours. After the reaction, the dichloromethane and excess sulfoxide chloride were concentrated under reduced pressure to get the intermediate 2, which was directly used for the next reaction [1].
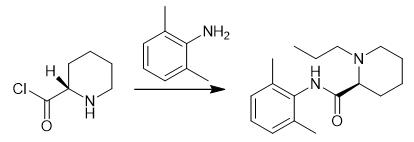
Fig. 4 The synthetic step 3 of Ropivacaine.
N, N-dimethylformamide (500 mL) and potassium hydroxide (81.92 g,1.46 mol) were added to the reaction flask of intermediate 2(111.77 g, 0.58 mol) obtained in the previous step, and stirred at room temperature for 1 hour. Add 2, 6-dimethylaniline (84.92 g, 0.70 mol) by drop, add to finish, temperature to 45℃ reaction for 2 hours, after the end of the reaction to room temperature, pour the reaction solution into 1200 mL ice water, precipitate a large amount of white solid, stir for 1 hour, filter cake with 800 mL water for 1 hour, filter, After drying at 60℃ for 10 hours, 148 g white solid was obtained with a yield of 92.4% [1].
Pharmacokinetics
In patients with chronic end-stage liver disease, the peak plasma concentrations of ropivacaine after a single intravenous ropivacaine dose are similar to those in healthy subjects. However, patients with end-stage liver disease have about a 60% lower mean ropivacaine clearance than healthy subjects and are thus expected to have over two-fold higher steady-state ropivacaine plasma concentrations during a continuous ropivacaine infusion. The peak plasma concentrations of ropivacaine after an axillary plexus block in uraemic patients are considerably higher than those in non-uraemic patients. However, uraemic patients have significantly higher alpha-1-acid glycoprotein plasma concentrations than non-uraemic patients, and the peak plasma concentrations of free ropivacaine (related to toxicity) are similar in both groups. The pharmacokinetics of intravenously administered ropivacaine in patients with renal insufficiency and the possibility of clinically significant (S)-2',6'- pipecoloxylidide metabolite accumulation during continuous or repeated ropivacaine administration in these patients remain to be clarified [2].
Pulpal anesthesia success rates for ropivacaine following maxillary infiltration anesthesia seem to be low. We investigated the hypothesis that the addition of epinephrine would affect the pharmacokinetics of ropivacaine by retaining ropivacaine in the mucosa of the injected area through the time-dependent distribution of ropivacaine in the rat maxilla and serum following maxillary infiltration anesthesia using (3)H-labeled ropivacaine. We then examined the vasoactivity of ropivacaine with or without epinephrine on local peripheral blood flow. The addition of epinephrine to ropivacaine increased ropivacaine concentrations in the palatal mucosa and adjacent maxilla by more than 3 times that of plain ropivacaine at 20 minutes. By observing the autoradiogram of (3)H-ropivacaine, plain ropivacaine in the maxilla was remarkably reduced 20 minutes after injection. However, it was definitely retained in the palatal mucosa, hard palate, adjacent maxilla, and maxillary nerve after the administration with epinephrine. Ropivacaine with epinephrine significantly decreased labial blood flow. This study suggests that 10 mug/mL epinephrine added to 0.5% ropivacaine could improve anesthetic efficacy and duration for maxillary infiltration anesthesia over plain ropivacaine [3].
Application
As a local anesthetic agent
Ropivacaine is a new amide-type local anesthetic agent, Unlike bupivacaine and mepivacaine, two structurally similar local anesthetic compounds, ropivacaine is exclusively the S-(-)-enantiomer. Ropivacaine is predominantly eliminated by extensive metabolism in the liver, with only 1% of the dose being excreted unchanged in the urine of humans, Four of the metabolites formed in human liver microsomes were identified as 3-OH-ropivacaine, 4-OH-ropivacaine, 2-OH-methyl-ropivacaine, and 2',6'-pipecoloxylidide (PPX). The enzymes involved in the human metabolism of ropivacaine have not been identified, To ascertain which forms of cytochrome P450 are involved, ropivacaine was incubated with human microsomes from 10 different livers having different cytochrome P450 activities. A strong correlation was found between the formation of 3-OH-ropivacaine and CYP1A (r = 0.87-0.89) and between the formation of 4-OH-ropivacaine, 2-OH-ropivacaine, and PPX and CYP3A (r = 0.97-1), Incubation of ropivacaine and human liver microsomes in the presence of alpha-naphthoflavone or furafylline, inhibitors of CYP1A, decreased the formation of 3-OH-ropivacaine by about 85%, without affecting the formation of the other metabolites, The formation of 4-OH-ropivacaine, 5-OH-methyl-ropivacaine, and PPX was markedly inhibited in the presence of troleandomycin, an inhibitor of CYP3A. Microsomes from cells expressing CYP1A2 formed 3-OH-ropivacaine, whereas 4-OH-ropivacaine, 2-OH-methyl-ropivacaine, and PPX were formed in microsomes from cells expressing CYP3A4, Inhibitors of CYP2C (sulfaphenazole), CYP2D6 (quinidine), and 2E1 (diethyldithiocarbamate) did not inhibit the formation of any metabolite from ropivacaine, In conclusion, CYP1A catalyzes the formation of 3-OH-ropivacaine, the main metabolite formed in vivo, whereas the formation of 4-OH-ropivacaine, 2-OH-methyl-ropivacaine, and PPX was catalyzed by CYP3A [4].
Ropivacaine is a new local anaesthetic with advantages that suggest an important role in the provi
You may like
See also
Lastest Price from Ropivacaine manufacturers

US $0.00/kg2025-04-22
- CAS:
- 84057-95-4
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg

US $0.00-0.00/kg2025-04-21
- CAS:
- 84057-95-4
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 900kg