The application of Bisabolol
General description
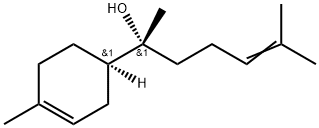
Bisabolol is a natural product found in Eupatorium altissimum, Psidium guajava, and other organisms with data available. Bisabolol, also known as Sweet Red Bisabolol and parthenol, is one of the sesquiterpene compounds in nature, huang Ju, Aspen, and certain essential oils of the genera Cynanchum and Salvia. Α-hong myrrh has many medicinal effects, such as anti-inflammation, sterilization, ulcer healing and gallstone dissolving, so it is widely used in pharmaceutical industry. Bisabolol is one of the important monocyclic sesquiterpenes, derived naturally from essential oils of many edible and ornamental plants. It was first obtained from Matricaria chamomilla, commonly known as chamomile or German chamomile[1].
Application and Pharmacology
Bisabolol protects and heals the skin from the effects of daily tension and accelerates the healing process, especially for sensitive skin and the body, widely used in personal care (skin and body lotions, aftershave and suncare, care products) formulations, along with its anti-inflammatory, natural, and safe properties, make it A, active ingredients commonly used in skin care. International only in personal care products, the application of the annual demand of more than 400t. Gradually become personal skin care raw materials new favorite. It has a light and pleasant aroma, and is also a stable fixative. Its application in flavor has been paid more and more attention. The available literature indicates that this plant along with other α-Bisabolol containing plants is popularly used in traditional medicine for potential health benefits and general wellbeing. Nutritional studies are indicative of the health benefits of α-Bisabolol. Numerous experimental studies demonstrated pharmacological properties of α-Bisabolol including anticancer, antinociceptive, neuroprotective, cardioprotective, and antimicrobia.
1.Alpha-bisabolol is a plant-derived sesquiterpene alcohol recently associated with a supposed anti-cancer action due to its ability to induce BID-related apoptosis. The molecule, which enters the cell through lipid rafts, may also interact with kisspeptin receptor 1, which has recently been associated with tumor mobility and invasiveness. This evidence suggests the possibility that α-bisabolol might act on the epithelial?mesenchymal transition mechanism, closely associated with the desmoplastic reaction of adipose tissue surrounding a pancreatic ductal adenocarcinoma.
2.Bisabolol was also found to enhance the effectiveness in attenuating pruritus and inflamed skin in patients with atopic dermatitis when combined with heparin in a topically applied formulation. Furthermore, an improvement was observed in the SCORing Atopic Dermatitis (SCORAD) values in patients who applied the combined therapy as compared with control and individualized therapy groups. On the other hand, the integration of α-Bisabolol in a formulation intended for the treatment of melasma has shown an enhancement in the recovered melasmatic area measured by imaging techniques at the end of treatment duration compared with baseline. Obtained results reported a statistical significance in patients’ satisfaction in terms of improved facial texture, skin oiliness, brightness, hydrated skin, and overall appearance following the treatment[2].
3.To elucidate the underlying cellular perturbations in MI to develop effective therapeutic agents, α-Bisabolol was investigated for its protective properties in MI induced by isoproterenol in rats. The study has provided substantial evidence on the vital role Bisabolol has in ameliorating MI damage. α-Bisabolol was found to be capable of de- creasing the level of serum lactate dehydrogenase (LDH), lipid peroxidation reflected by decreased amount of thiobarbituric acid reactive substances (TBARS) as well as myocardial infarct size. α-Bisabolol also exhibited an antioxidant effect manifested by elevating the activity of both SOD and CAT[2].
4.The antimicrobial activity of α-Bisabolol was evaluated against Escherichia coli, Staphylo-coccus aureus, Candida albicans, Candida krusei, Candida tropicalis, and multi-resistant bacterial strains, Staphylococcus aureus and Escherichia coli. Results showed that all strain were sensitive to α-Bisabolol which also exerted a synergism when applied with aminoglycosides.In the same manner, α-Bisabolol demonstrated an antibacterial effect against Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa as well as a synergism against S. aureus, when combined with the antibiotic norfloxacin and against E. coli when combined with gentamicin. In digging into the antibacterial mechanisms associated with α-Bisabolol, α-Bisabolol was tested for a potential inhibition of TetK and NorA efflux pump responsible for pumping out antibiotics into extracellular space and thus rendering the bacteria resistant to the administered antibiotic. The combination of α-Bisabolol with tetracycline and norfloxacin resulted in potentiation of their action, illustrated by a reduction in their minimum inhibitory concentration (MIC) from 192 μg/mL to 128 μg/mL against SA IS-58 TetK pump expressing strain and from 256 μg/mL to 32 μg/mL against SA 1199 B NorA pump expressing strain, respectively.
Synthesis
With the increase in demand, the amount of pure natural extraction seems unable to meet the market demand. In this paper, a high value-added α-red myrrh alcohol was prepared from dipentene (by-product of finished product, which was made from turpentine from Guangxi province)
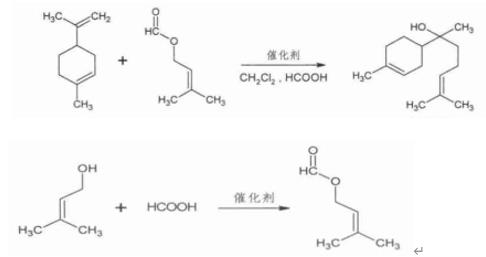
Figure 1. the synthesis route of Bisabolol
In a 500L flask equipped with a thermometer and a tetrafluoroethene agitator, isoamyl alcohol was added to excess formic acid (molar ratio = 1∶1.5) , and esterified to obtain isoamyl alcohol formate, gC detection tracks reaction endpoints. After the esterification reached the end point, dipentamyl, olefins and dichloromethane were added to the reaction system, and the condensation reaction was stirred at 50-70 a ° C by dropping the catalyst. The solvent was recovered and the dichloromethane ofBisabolol was obtained. Bisabolol was prepared from dipentene and prenol by esterification and condensation reaction. The optimum conditions of esterification reaction were as follows: 35 ° C, Prenol and formic acid mole ratio 1∶1.5, reaction time 6 hours, the yield was 92% . In the condensation stage, A3B3C2D3 was selected as the optimum process, i. e. 50% solvent, 7% catalyst, 70 ° C temperature, prenol, 1∶4 mole ratio of formate to dipentene. The yield was 68.0%[3].
Safety
Bisabolol was evaluated for genotoxicity, repeated dose toxicity, developmental and reproductive toxicity, local respiratory toxicity, phototoxicity/photoallergenicity, skin sensitization, and environmental safety. Data show that α-bisabolol is not genotoxic. Data on α-bisabolol provide a calculated margin of exposure (MOE) > 100 for the repeated dose toxicity and developmental toxicity endpoints. The reproductive and local respiratory toxicity endpoints were evaluated using the threshold of toxicological concern (TTC) for a Cramer Class I material, and the exposure to α-bisabolol is below the TTC (0.03 mg/kg/day and 1.4 mg/day, respectively). Data from α-bisabolol provided a No Expected Sensitization Induction Level of 5500 μg/cm2 for the skin sensitization endpoint. The phototoxicity/photoallergenicity endpoints were evaluated based on data and ultraviolet (UV) spectra; α-bisabolol is not expected to be phototoxic/photoallergenic. The environmental endpoints were evaluated; α-bisabolol was found not to be persistent, bioaccumulative, and toxic (PBT) as per the International Fragrance Association Environmental Standards,
You may like
Related articles And Qustion
See also
Lastest Price from alpha-Bisabolol manufacturers

US $0.00-0.00/KG2025-07-09
- CAS:
- 515-69-5
- Min. Order:
- 1KG
- Purity:
- 99% up by HPLC
- Supply Ability:
- 20TONS

US $10.00/KG2025-04-21
- CAS:
- 515-69-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt