Spotlight on N-Octyl Pyrrolidone: Antibacterial Activity, Environmental Fate, and Solvent Effects
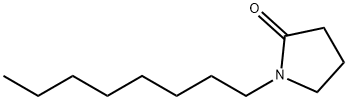
1-Octylpyrrolidin-2-one or N-Octyl pyrrolidone (NOP) is a member of the N-alkylpyrrolidin-2-one group, which comprises important industrial compounds applicable to several spheres of human activity. N-Octyl pyrrolidone is considered a very weak base, proving to be a useful surfactant due to the chemical bonding of a non-polar alkyl chain with a hydrophilic pyrrolidin-2-one head; its hydrophilic-lipophilic balance equals 6. It is stable to photolytic degradation in water and soil, and its logKow is 4.15.

Degradation of antibacterial N-Octyl pyrrolidone
N-Octyl pyrrolidone is soluble in other non-ionic surfactants, slightly soluble in water (1.2 g L−1) and possesses both chemical and thermal stability (Login 1995). A further advantage is its high solvency of hydrophobic molecules; hence, it supremely combines surface-active and solvent properties. It is applied as a solvent and wetting agent, a chemical intermediate, and a permeation enhancer for transdermal drug delivery. The antibacterial properties of N-Octyl pyrrolidone were partially described by Kabra, who demonstrated that a concentration of 500 mg L−1 supplemented with 18 g L−1 propylene glycol killed both Gram-negative (Pseudomonas aeruginosa and Escherichia coli) and Gram-positive (Staphylococcus aureus) bacteria in 6 h. Due to its properties and application extent, N-Octyl pyrrolidone may enter different environmental spheres, either as component of the several agrochemicals or as a result of improper handling during its storage, transport and actual use. Although it revealed to be biodegradable in screening water test, there has been no study describing NOP-degrading microorganisms from various environmental spheres so far. Hence, this study set out to obtain such bacterial members from two main environmental spheres, river water and alluvial soil and to describe their roles in the compound degradation.[1]
N-Octyl pyrrolidone demonstrated bacteriostatic and bactericidal effects on test species of Gram-positive and Gram-negative bacteria except for pseudomonads. The comparison of the concentration range of antibacterial properties of NOP and its both maximum growth concentration (175 mg L−1) and MIC values for primary degraders (250 mg L−1) does not indicate any special resistance of the strains against the compound. Only B. petrii R1 revealed significantly higher tolerance towards N-Octyl pyrrolidone. Thus, the capability of octane-utilising phenylobacteria to recognise the octyl chain of NOP seems to be a crucial agent for bacterial NOP degradation under aerobic condition. As detailed tandem mass spectrometry analysis showed that 4-(2-oxopyrrolidin-1-yl)butanoic acid is the intermediate released by primary degraders after their growth on NOP, it is evident that the obtained phenylobacteria use only four carbon atoms of N-Octyl pyrrolidone.
The study of bacterial degradation of N-Octyl pyrrolidone (NOP) by river water and soil bacteria was the main aim of the research. Although the compound demonstrated bacteriostatic as well as bactericidal effects against Gram-positive and certain Gram-negative bacteria at concentrations ranging from 100 to 1000 mg L−1, its concentration of 100 mg L−1 was successfully degraded by microbial communities of both river water and alluvial soil; removal efficiencies reached 87.2 and 88.4% of dissolved organic carbon, respectively. Isolation of the strains responsible for the process showed that bacterial degradation was initiated by the octane-utilising bacteria of the genus Phenylobacterium, which used four carbon atoms of the N-Octyl pyrrolidone octyl chain and oxidised terminal carbon atom of the remaining chain. The structure of the intermediate produced by phenylobacteria was elucidated following the results obtained from the detailed electrospray mass spectrometry (ESI–MS) analysis; these experiments showed that it is a 4-(2-oxopyrrolidin-1-yl)butanoic acid.
Effects of Electrolyte Concentration on the Rotational Dynamics of Resorufin
Scientists report on the rotational diffusion dynamics of the anionic chromophore resorufin in water and N-Octyl pyrrolidone (NOP) solutions as a function of solution electrolyte concentration. Our data show that resorufin exhibits a single exponential anisotropy decay in aqueous solutions containing up to 0.1 M lithium perchlorate (LiClO4). In contrast to the observed behavior of resorufin in pure N-Octyl pyrrolidone, where biexponential decay occurs, we also observe a single exponential anisotropy decay for resorufin in NOP with the addition of up to 0.1 M tetrabutylammonium bromide (TBAB) or tetraoctylammonium bromide (TOAB). For resorufin in N-Octyl pyrrolidone, the reorientation time constant increases with increasing electrolyte concentration, consistent with complexation between the resorufin anion and the electrolyte ammonium cation. We observe a qualitatively different trend in the aqueous resorufin solutions and understand these data for both solvent systems in the context of interactions between the chromophore and cationic species present.[2]
The work we present here addresses the role of electrolyte (ionic species) in mediating the rotational diffusion behavior of resorufin in two different solvent systems, water and N-Octyl pyrrolidone. The local environment of resorufin in NOP is characterized by a chromophore local environment determined substantially by solvent−solvent interactions, while the second system, resorufin in water, manifests a chromophore local environment where solvent−solute interactions play an important role. Resorufin reorients as a prolate rotor, exhibiting a single exponential anisotropy decay in both water and N-Octyl pyrrolidone when electrolyte is present in the solution. This behavior represents a change in the effective rotor shape of resorufin in NOP with the addition of electrolyte. The three electrolytes used here are LiClO4, tetrabutylammonium bromide (TBAB), and tetraoctylammonium bromide (TOAB).
Resorufin is known to exhibit single exponential anisotropy decay dynamics in water and a two-component anisotropy decay in N-Octyl pyrrolidone. In that work, we found that solvent−solvent interactions between N-Octyl pyrrolidone molecules dominated the environment formed around the resorufin chromophore, mediating its reorientation dynamics by enforcing rotation primarily about the axis perpendicular to the chromophore π-system plane. The addition of electrolyte molecules with hydrodynamic volumes larger than that of the solvent affects solvent organization and also gives rise to ionic interactions between the electrolyte and the chromophore, resulting in a change in the effective rotor shape of resorufin. The reorientation times of resorufin in water and N-Octyl pyrrolidone exhibit an electrolyte concentration dependence.
Solvent-Dependent Changes in Molecular Reorientation Dynamics
Researchers report on the rotational diffusion dynamics of two different chromophores, resorufin and 6-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)hexanoic acid (NBDHA) in water and N-Octyl pyrrolidone (NOP) solvents. We measure the induced orientational anisotropy function, R(t), using time-correlated single photon counting. Our data show that both chromophores exhibit single exponential anisotropy decays in aqueous solution and two-component exponential anisotropy decays in N-Octyl pyrrolidone. The change of the anisotropy decay functionality indicates that the effective rotor shape swept out by solute rotational motion is different in the two solvents. We interpret these findings in the context of Chuang and Eisenthal’s theory of fluorescence depolarization by rotational diffusion. The similarity in the behavior of the two different chromophores in these solvent systems points to solvent−solvent interactions and local organization as the dominant factors in mediating motional dynamics.[3]
Scientists have examined the steady-state and time-resolved spectroscopic behavior of two chromophores, resorufin and NBDHA, in the solvents water and N-Octyl pyrrolidone. The steady-state spectroscopic data point to significantly different solvent−solute interactions for the two chromophores in the different solvents, with resorufin exhibiting a different change in dipole moment upon excitation for the two solvents and NBDHA not exhibiting the analogous change. For reorientation in N-Octyl pyrrolidone, a different rotor shape is observed, but both chromophores behave in the same manner, exhibiting an oblate rotor shape. The change in rotor shape is correlated with the increase in the hydrodynamic volume of the solvent relative to the solutes. Because of the similarity of the dynamics for the two chromophores, solvent−solvent interactions are implicated as the mediating factor in determining the local solvation environments that are allowed to form in this solvent
References
[1]Ruzicka J, Julinova M, Rouchal M, Salac J, Vanharova L, Urban J, Pancochova K. Degradation of antibacterial 1-octylpyrrolidin-2-one by bacterial pairs isolated from river water and soil. Environ Sci Pollut Res Int. 2022 Jun;29(30):45292-45302.
[2]Hay CE, Marken F, Blanchard GJ. Effects of electrolyte concentration on the rotational dynamics of resorufin. J Phys Chem A. 2010 Dec 16;114(49):12875-80.
[3]Hay CE, Marken F, Blanchard GJ. Solvent-dependent changes in molecular reorientation dynamics: the role of solvent-solvent interactions. J Phys Chem A. 2010 Apr 15;114(14):4957-62.
You may like
Lastest Price from N-Octyl pyrrolidone manufacturers

US $10.00/kg2025-04-21
- CAS:
- 2687-94-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $6.00/kg2025-04-21
- CAS:
- 2687-94-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month