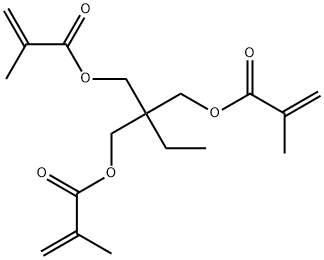
Trimethylolpropane Trimethacrylate: From Industrial Preparation to Mechanical Performance
Trimethylolpropane trimethacrylate is a multifunctional monomer with a wide range of industrial applications as a cross-linking agent,reactive diluent,and chemical intermediate. Trimethylolpropane trimethacrylate is a premium quality substance that is mainly consumed as a precursor to alkyd resins,high-gloss coatings,and ion exchange resins.

Preparation of Trimethylolpropane trimethacrylate
1775 kg of trimethylolpropane, 1018 kg of methyl methacrylate (MMA) and also 1433 kg of recycled MMA from Comparative Example 1, 0.123 kg of hydroquinone monomethyl ether as inhibitor and a mixture of 10 kg of calcium oxide and 2.5 kg of lithium chloride as catalyst are combined in a 6 m3 stirred tank reactor provided with agitator, steam heating, distillation column and condenser and the mixture is stirred while passing in air. To stabilize the column, a total of 151 kg of MMA containing 0.12 kg of hydroquinone monomethyl ether and 0.016 kg of 4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl in dissolved form are introduced into the column runback during the course of the reaction. The apparatus is heated to a temperature at the bottom of 100° C., with the column initially being operated under total reflux. As soon as the temperature at the top of the column drops below 70° C., the methanol/MMA mixture is taken off at a reflux ratio of 4:1. The MMA stock in the reactor is supplemented by introduction of equal parts of fresh MMA per part of methanol/MMA mixture taken off. A total of 1414 kg of MMA are in this way introduced over a period of 5 hours. Over a period of 8 hours, the reflux ratio is adapted to the decreasing formation of methanol to 27:1. A total of 1410 kg of methanol/MMA mixture are discharged. Above a temperature at the top of the column of 85° C., the methanol/MMA mixture is low in methanol and is collected in a separate vessel for reuse as raw material in the next batch. At a temperature at the bottom of 115° C., the reaction is complete and excess MMA is taken off under reduced pressure, with the pressure gradually being reduced to 100 mbar. When no more MMA distills off, the vacuum is broken. The contents of the tank, which comprise the catalyst-containing rimethylolpropane trimethacrylate, are admixed with 18 kg of bleaching earth and 12 kg of aluminium silicate (Perlite) as filter aid and freed of the catalyst by washcoat filtration. The filtrate is fed into a continuous evaporator (area: 3.5 m2) having a rotating wiper system at a pressure of 18 torr and an evaporator temperature of 134° C. A total of 1830 kg of trimethylolpropane trimethacrylate are obtained from the bottom product. [1]
Multifunctional Acrylate's Addition to Methacrylate
This study investigated the effects of a multifunctional acrylate copolymer—Trimethylolpropane Triacrylate (TMPTA) and Di-pentaerythritol Polyacrylate (A-DPH)—on the mechanical properties of chemically polymerized acrylic resin and its bond strength to a CAD/CAM polymethyl methacrylate (PMMA) disk. The methyl methacrylate (MMA) samples were doped with one of the following comonomers: TMPTA, A-DPH, or Trimethylolpropane Trimethacrylate (TMPTMA). The doping ratio ranged from 10 wt% to 50 wt% in 10 wt% increments. The flexural strength (FS) and modulus (FM) of PMMA with and without comonomer doping, as well as the shear bond strength (SBS) between the comonomer-doped PMMA and CAD/CAM PMMA disk, were evaluated. The highest FS (93.2 ± 4.2 MPa) was obtained when doped with 20 wt% of TMPTA. Trimethylolpropane trimethacrylate with a methacryloyl group and TMPT with an acryloyl group showed a similar biphasic behavior in terms of the SBS values as the doping ratio increased, while the addition of A-DPH showed a monophasic response with a peak at 10 wt% addition. In other words, the comonomer’s addition did not necessarily improve the mechanical properties of acrylic resin even with an increased amount added. Another concern lies with the low water resistance of acrylates. Further studies are needed to examine the water absorption behavior and dissolution rate of acrylates.[2]
For Trimethylolpropane trimethacrylate, the FS decreased with the increase in the doping ratio. For SBS, TMPTA showed almost constant values (ranging from 7.0 to 8.2 MPa) regardless of the doping amount, and A-DPH peaked at 10 wt% doping (8.7 ± 2.2 MPa). Trimethylolpropane trimethacrylate showed two peaks at 10 wt% (7.2 ± 2.6 MPa) and 40 wt% (6.5 ± 2.3 MPa). Regarding the failure mode, Trimethylolpropane trimethacrylate showed mostly adhesive failure between the CAD/CAM PMMA disk and acrylic resin while TMPTA and A-DPH showed an increased rate of cohesive or mixed failures. Acrylate’s addition as a comonomer to PMMA provided improved mechanical properties and bond strength to the CAD/CAM PMMA disk.
Wear characteristics of trimethylolpropane trimethacrylate filler-containing resins
Although the demand for aesthetic restoration of primary molars has increased, the full-crown restorations using resin and the details of the wear characteristics of trimethylolpropane trimethacrylate (TMPT) filler containing resins for primary molars are not well understood. This study was conducted to determine whether new light-cured composite resin (Fantasista) and 4-META/MMATBB resin (Bondfill SB) are appropriate for full crown restoration of primary molars by evaluating their wear characteristics. Both resins products contain TMPT filler. The properties of the resins were evaluated through in vitro impacting-sliding wear tests; the wear properties of the opposing enamel specimens used in the tests were also studied. The properties of the resins were compared with those of Litefill, MetafilC, and Clearfil FII, which had been evaluated previously. Fantasista exhibited simple shape of wear that was suggestive of a higher wear resistance than that of Litefill. Fantasista caused the least damage to the antagonistic primary enamel.[3]
References
[1]EVONIK ROEHM - US2010/204509, 2010, A1
[2]Maruo Y, Yoshihara K, Irie M, Nagaoka N, Matsumoto T, Minagi S. Does Multifunctional Acrylate's Addition to Methacrylate Improve Its Flexural Properties and Bond Ability to CAD/CAM PMMA Block? Materials (Basel). 2022 Oct 28;15(21):7564.
[3]Wada K, Ikeda E, Wada J, Inoue G, Miyasaka M, Miyashin M. Wear characteristics of trimethylolpropane trimethacrylate filler-containing resins for the full crown restoration of primary molars. Dent Mater J. 2016;35(4):585-93
You may like
Lastest Price from Trimethylolpropane trimethacrylate manufacturers

US $1.10/g2025-09-15
- CAS:
- 3290-92-4
- Min. Order:
- 1g
- Purity:
- 99.9%
- Supply Ability:
- 100 Tons Min

US $1.10/g2025-09-13
- CAS:
- 3290-92-4
- Min. Order:
- 1g
- Purity:
- 99.9%
- Supply Ability:
- 100 Tons Min