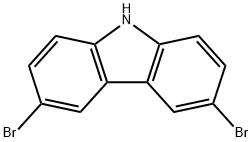
3,6-Dibromocarbazole: Environmental Behavior, Anticancer Potential, and Microplastic Sorption Research
3,6-Dibromocarbazole is used as a pharmaceutical intermediate and also an essential intermediate for synthesizing optoelectronic materials. It is the essential intermediate of synthesis of N-alkylation bromo carbazole compounds and is used to prepare N-(2-hydroxyethyl)-3,6-dibromocarbazole.

Effects of 3,6-Dibromocarbazole on Soil Health
Polyhalogenated carbazoles (PHCZs) are compounds in which the hydrogen atoms in the carbazole ring are replaced by halogen atoms. In the past 5 years, PHCZs have been detected widely in multiple environmental media and have drawn increasing attention as a type of emerging contaminants. Among PHCZs detected in the environment, 3,6-dibromocarbazole (36-DBCZ) has a very high detection rate and concentration. Wu et al. identified 11 PHCZ congeners with a total concentration of up to 15.8 ng/g dw in the sediment of Lake Tai. Among them, 36-DBCZ was the dominant one of relative abundance. Also, 3,6-Dibromocarbazole was the predominant PHCZ congener in drinking water. Mumbo et al. reported that the highest concentrations of 3-CCZ, 36-DCCZ, and 36-DBCZ were 9.1, 19.8, and 67.6 ng/g, respectively, in the forest soil of Germany. Mumbo et al. also reported that 36-DBCZ’s half-life in soil is 120 days, and its Log Kow value was 5.34, indicating that 36-DBCZ is persistent in soil and easily bio-accumulated. In the present study, the effects of 3,6-Dibromocarbazole on soil enzyme activity and the soil microbial functional diversity were investigated. This study gives new insight and provides valuable data for the risk assessment of PHCZs.[1]
The effects of 3,6-Dibromocarbazole on soil enzyme activities are shown. Generally speaking, the soil enzymes were mainly affected at the beginning (the first 20 days) of 36-DBCZ exposure, then the influences gradually faded out as time went on. This phenomenon might be attributed to the degradation of 36-DBCZ in soil. Although the residual dynamics of 36-DBCZ were not investigated in this study, Mumbo et al. have estimated the degradation half-life of PHCZs with two halogen atoms in the soil was about 120 days based on the PBT profiler. The results of the present study reflect the half-life of 3,6-Dibromocarbazole in the test soil might be shorter than that estimation. The exact data should be investigated in future study. Consistent with the response of soil dehydrogenase activity, the Biolog-ECO assay also revealed that the soil microbial activity was markedly decreased under the 3,6-Dibromocarbazole exposure on the 3rd and 10th days. Average well color development (AWCD) is a comprehensive indicator to reflect the microbial activity, which reflects the overall utilization of different carbon sources. As shown, the AWCD lines in 36-DBCZ-treated groups were markedly lower than that of the control on the 3rd and 10th days, indicating the 36-DBCZ inhibited the activity of the soil microbe. Furthermore, the inhibition increased as the concentration went up on the 3rd day. As time went by, the inhibitions disappeared on the 20th and 40th days. After that an increase of microbial activity was found in 3,6-Dibromocarbazole -treated groups on the 80th, and 120th days. We can see that the AWCD values of the control group decreased markedly on the 40th, 80th, and 120th days than those on the 3rd, 10th, and 20th days.
The present study performed a preliminary investigation of 3,6-dibromocarbazole (a PHCZ with a high detection rate, and concentration in the environment) at concentrations of 0.1, 1.0, 10, and 100 mg/kg on the soil health, based on soil enzyme test and Biolog-ECO assay. Results showed that 36-DBCZ could inhibit the activity and diversity of soil microbes, even at the environment-relevant concentration (0.1 mg/kg). But, the inhibition lasted only about 10 days. As time passed, slight increases in microbe activity and diversity were found in 36-DBCZ-treated groups. We hypothesized that the degradation products of 3,6-Dibromocarbazole provided extra nutrients to the soil microbes, which required further verification. Activities of urease, β-glucosidase, and acid phosphatase were increasingly increased, in contrast to the microbial activity. The present study provides valuable data on the effects of PHCZs on the soil ecosystem, and we suggest that the degradation of PHCZs, as well as their influences on the structure and functions of the soil microbial community, should be investigated in future studies.
Synthesis, Anti-Cancer and Anti-Migratory Evaluation of 3,6-Dibromocarbazole
The aim of this study is to design and synthesize a new series of N-alkyl-3,6-dibromocarbazole and N-alkyl-5-bromoindole derivatives and analyze their cytotoxic effect and potential to inhibit actin cytoskeleton rearrangement and cancer cell migration. The structural elements of Wiskostatin and derivatives identified as pharmacophoric unit are a 3,6-dihalogen carbazole and a dialkylamino-2-propanol chain. Our strategy was to design and synthesize a new series of compounds with a 3,6-dibromocarbazole or 5-bromoindole ring connected, via a three-carbon atom aliphatic chain, to an amide group. The influence of different N-alkyl or aromatic substituents at the amide group was examined.[2]
The synthetic method to construct the 3,6-dibromocarbazole derivatives library is described. The N-alkyl-3,6-dibromocarbazole derivatives were generated in a three-step synthesis using 3,6-dibromocarbazole as origin of the carbazole derivatives core. Compound 1 was reacted with ethyl 4-bromobutyrate to introduce the aliphatic side chain by nucleophilic substitution followed by hydrolysis to afford the corresponding 3,6-diromocarbazole-4-butyric acid. For the generation of 3,6-dibromocarbazole-4-butyramide derivatives, compound was therefore used as starting material, which reacted with different amines via an amide coupling reaction using N-(3-Dimethylaminopropyl)-N’-ethylcarbonate (EDAC) with Hydroxybenzotriazole (HOBt) as an additive dissolved in methylene chloride (CH2Cl2). From the twenty-four compound derivatives of 3,6-dibromocarbazole, it can be observed that in the MCF-7 (ER+) cancer cell line, compounds showed good to moderate antiproliferative activity with a GI50 in the range of 6.8–32.2 µM. In general, these results suggest that new N-alkyl-3,6-dibromocarbazole and compounds derivatives of Wiskostatin can be explored as new probes to study actin dynamics in cancer cells, or to further develop new anti-cancer and anti-metastatic drugs.
Sorption of 3,6-dibromocarbazole and 1,3,6,8-tetrabromocarbazole by microplastics
Microplastics and organic pollutants are typical contaminants in the marine environment. However, little is known about their interactions. In this study, the sorption of 3,6-Dibromocarbazole(3,6-BCZ) and 1,3,6,8-Tetrabromocarbazole (1,3,6,8-BCZ) by Polypropylene microplastic in simulated seawater was studied. Factors, including particle size, salinity and concentration, were investigated, and the experimental results were simulated using a mathematical model. Results showed that the pseudo-second-order kinetic model was more suitable to describe the sorption of polyhalogenated carbazole by microplastics, with equilibrium sorption times of 6 h and 8 h for 3,6-dibromocarbazole and 1,3,6,8-BCZ, respectively. Sorption capacity increased with decreasing particle size and the adsorption capacity increased initially and then decreased with increasing salinity, with a maximum sorption occurring at salinity of 14%. Moreover, the sorption amount increased with the increasing concentration of polyhalogenated carbazole. The sorption isotherms were confirmed as the extended Langmuir model and the extended Freundlich model, both of which were S-type.[3]
The maximum adsorption rate is about 54% and 57% for 3,6-Dibromocarbazole and 1,3,6,8-BCZ, respectively, when the salinity is 14%. The adsorption rate decreases with increasing salinity when NaCl concentration is >14%. It is not clear what causes this phenomenon, the reason of which may be the adsorption competition of NaCl. Also shown, the adsorption of 3,6-Dibromocarbazole is lower than 1,3,6,8-BCZ when the salinity is less than approximately 9%, while the adsorption of 3,6-BCZ is >1,3,6,8-BCZ when salinity is >9%. Further studies need to be carried out to research this phenomenon. The effect of different adsorption concentrations on the adsorption of 3,6-BCZ and 1,3,6,8-BCZ by PP at the initial concentration of 50 μg/L after 8 h is shown. The effect of adsorption concentration on adsorption is very clear: the adsorption capacity appears to increase linearly with increasing adsorption concentration.
References
[1]Du, Z., Zhang, J., Cheng, C. et al. Effects of 3,6-Dibromocarbazole on Soil Health—Based on Soil Enzymes and the Biolog-ECO Test. Water Air Soil Pollut 233, 256 (2022).
[2]Butler-Fernández KM, Ramos Z, Francis-Malavé AM, Bloom J, Dharmawardhane S, Hernández E. Synthesis, Anti-Cancer and Anti-Migratory Evaluation of 3,6-Dibromocarbazole and 5-Bromoindole Derivatives. Molecules. 2019 Jul 24;24(15):2686.
[3]Zhang X, Zheng M, Yin X, Wang L, Lou Y, Qu L, Liu X, Zhu H, Qiu Y. Sorption of 3,6-dibromocarbazole and 1,3,6,8-tetrabromocarbazole by microplastics. Mar Pollut Bull. 2019 Jan;138:458-463.
Lastest Price from 3,6-Dibromocarbazole manufacturers
US $0.00/kg2025-04-21
- CAS:
- 6825-20-3
- Min. Order:
- 1kg
- Purity:
- 99
- Supply Ability:
- 100kg

US $30.00-10.00/KG2025-04-15
- CAS:
- 6825-20-3
- Min. Order:
- 50KG
- Purity:
- 99%
- Supply Ability:
- 500000kg