Sodium Thiosulfate: An Essential Compound in Chemistry and Industry
Introduction
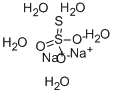
Sodium thiosulfate (Na₂S₂O₃) is an inorganic compound that has garnered substantial interest in both research and practical applications. It is most commonly encountered as its pentahydrate form (Na₂S₂O₃·5H₂O), a colorless, crystalline solid that readily dissolves in water. Due to its high reactivity and ability to engage in redox reactions, sodium thiosulfate is employed in various chemical processes.
Its unique chemical characteristics and numerous uses make it an indispensable substance in areas ranging from photographic processing to medical treatments and industrial water purification. This article delves into its nature, composition, and relevance across various domains.

Figure 1 Characteristics of SODIUM THIOSULFATE
Chemical Properties of Sodium Thiosulfate
At the molecular level, sodium thiosulfate consists of two sodium ions (Na⁺) and a thiosulfate anion (S₂O₃²⁻). The thiosulfate anion is structurally similar to sulfate (SO₄²⁻) but with one oxygen atom replaced by a sulfur atom. This substitution significantly alters the reactivity and properties of the compound.
Molecular Formula: Na₂S₂O₃
Molecular Weight: 158.11 g/mol (for anhydrous form)
Appearance: Sodium thiosulfate typically appears as a white or colorless crystalline solid in its pentahydrate form, Na₂S₂O₃·5H₂O. It is odorless and highly soluble in water, with a solubility of 70.1 g/100 mL at 20°C.
Sodium thiosulfate is stable at room temperature, though it tends to decompose when heated, releasing sulfur dioxide gas (SO₂) and sulfur, both of which are reactive byproducts.
In an aqueous solution, sodium thiosulfate acts as a weak base. The compound is known for its ability to undergo redox reactions, particularly with iodine, making it a common reagent in titrations for determining iodine concentration (iodometry).
Main Components
The main components of sodium thiosulfate include:
Sodium (Na): This alkali metal plays a vital role in stabilizing the thiosulfate anion by forming ionic bonds. It enhances the solubility of the compound in water and allows sodium thiosulfate to interact with a wide range of substances.
Thiosulfate (S₂O₃²⁻): The thiosulfate ion is a sulfur-oxygen anion that exhibits unique reactivity, especially in redox chemistry. This ion acts as both a reducing and oxidizing agent under different conditions, which makes sodium thiosulfate a versatile chemical in industrial and laboratory applications.
Applications of Sodium Thiosulfate
Sodium thiosulfate has a broad spectrum of uses across many industries, including medical, photographic, water treatment, and analytical chemistry.
1. Medical Uses
One of the most critical applications of sodium thiosulfate is in the medical field. The compound is an essential antidote for cyanide poisoning. Sodium thiosulfate reacts with cyanide to form thiocyanate, a much less toxic compound that is excreted in urine.
Additionally, sodium thiosulfate is used in the treatment of certain calciphylaxis, a serious condition affecting patients with kidney disease, where calcium deposits form in the small blood vessels of the skin.
2. Photographic Processing
In the traditional photography industry, sodium thiosulfate is widely used as a "fixer" in the development of black-and-white photographs. After exposure and development, sodium thiosulfate dissolves unreacted silver halides from the film or photographic paper, preventing further exposure and ensuring the image remains stable.
3. Analytical Chemistry
Sodium thiosulfate is an essential reagent in iodometric titrations. In such titrations, it serves as a reducing agent that reacts with iodine to produce iodide ions, thus allowing chemists to determine the concentration of iodine or other substances in a solution.
This property is crucial in water quality testing, where sodium thiosulfate is employed to measure chlorine levels in swimming pools, tap water, and industrial processes. Its ability to neutralize chlorine also extends to its use in aquariums and fish tanks to detoxify chlorinated water.
4. Water Treatment
In the water treatment industry, sodium thiosulfate dechlorinate water after chlorination. Chlorine, used to disinfect drinking water, can pose health risks if present in high concentrations. Sodium thiosulfate effectively neutralizes excess chlorine, making the water safe for consumption and use.
5. Gold Extraction
Sodium thiosulfate is also employed as an alternative to cyanide in gold-leaching processes. Thiosulfate leaching offers an eco-friendly and less toxic method for extracting gold from ores, particularly important in countries where cyanide usage is restricted due to environmental concerns.
Storage Methods of Sodium Thiosulfate
Proper storage of sodium thiosulfate is essential to maintain its effectiveness and stability. The compound, especially in its pentahydrate form, can degrade if exposed to moisture, air, or excessive heat. To ensure long-term storage and prevent decomposition, certain precautions should be followed:
Dry and Cool Environment: Sodium thiosulfate should be stored in a dry, cool place, away from sources of moisture and direct sunlight. Excessive heat can cause the compound to decompose, releasing sulfur dioxide gas.
Airtight Containers: Due to its hygroscopic nature (ability to absorb moisture from the air), sodium thiosulfate should be kept in airtight containers. Exposure to moisture may result in the gradual formation of a solution, potentially altering its reactivity.
Separation from Acids: Sodium thiosulfate should be stored away from strong acids, as it can react with acids to release sulfur dioxide, which is both toxic and corrosive.
Conclusion
Sodium thiosulfate is a chemical compound with a diverse range of applications that highlight its importance in various industries. From treating cyanide poisoning to fixing photographic images and neutralizing chlorine in water treatment, sodium thiosulfate plays a vital role in modern chemistry.
Its versatile nature is supported by its ability to participate in redox reactions, making it an essential reagent in analytical chemistry. Proper storage and handling of sodium thiosulfate ensure its longevity and efficacy, maintaining its value across medical, industrial, and laboratory applications.
As research into greener technologies continues, sodium thiosulfate’s role in areas such as gold extraction and water treatment will likely expand, further solidifying its status as an indispensable chemical in today’s world.
References:
[1] MELVIN R HAYDEN D J A G. Sodium thiosulfate: new hope for the treatment of calciphylaxis.[J]. Seminars in Dialysis, 2010, 23 3. DOI:10.1111/j.1525-139X.2010.00738.x.[2] MELVIN R HAYDEN D J A G. Sodium thiosulfate: new hope for the treatment of calciphylaxis.[J]. Seminars in Dialysis, 2010, 23 3. DOI:10.1111/j.1525-139X.2010.00738.x.
See also
Lastest Price from Sodium thiosulfate pentahydrate manufacturers

US $0.00/Kg/Drum2025-04-21
- CAS:
- 10102-17-7
- Min. Order:
- 25KG
- Purity:
- 99%-100.5%;USP
- Supply Ability:
- 200 TONS

US $10.00/KG2025-04-21
- CAS:
- 10102-17-7
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt