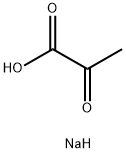
Sodium pyruvate:a crucial role in cellular metabolism
Introduction
Sodium pyruvate, the sodium salt of pyruvic acid serves a crucial role in cellular metabolism as a key intermediate in glycolysis and the citric acid cycle. Widely used in cell culture, it provides an additional energy and carbon source for in vitro cell growth. In the presence of oxygen, pyruvate can enter the mitochondria, participating in the citric acid cycle and contributing to ATP production. Beyond its energy-providing function, sodium pyruvate is recognized for its potential antioxidant properties, offering cellular protection. Stable under normal storage conditions, it is commonly supplied as a powder for easy dissolution in cell culture media. Apart from cell culture applications, sodium pyruvate finds use in various biochemical and molecular biology experiments, with researchers optimizing concentrations based on specific cell types and experimental requirements.
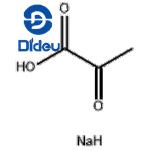
Figure 1 Sodium pyruvate, the sodium salt of pyruvic acid serves a crucial role in cellular metabolism
Application
The growth of cells requires a nutrient environment, and the nutrient matrix used to maintain cell growth is called a culture medium, which generally contains carbohydrates, nitrogen-containing substances, inorganic salts (including trace elements), vitamins, and water. In fact, most cells in the human body only use glucose, and sodium pyruvate is a necessary substance in cell culture. Sodium pyruvate acts as a substitute for carbon sources in culture media and participates in cellular nutrient metabolism. Sodium pyruvate is a type of endogenous small molecule substance. Both sodium pyruvate and pyruvate are naturally present in the human body and are necessary for metabolic and life activities. It is an energy substance. In cell culture experiments conducted in scientific research, scientific researchers have also discovered substances with similar effects, such as glucose and sodium lactate, which participate in metabolic activities and provide energy for human action. The role of sodium pyruvate in cell culture: Sodium pyruvate is necessary for metabolic and life activities in human daily life. It is an energy substance. In cell culture experiments conducted by scientific research, researchers have also discovered substances with similar effects, such as glucose and sodium lactate, which participate in metabolic activities and provide energy for human action, In the human body, most cells only use glucose, and sodium pyruvate is a necessary substance in cell culture. Sodium pyruvate acts as a substitute for carbon sources in culture media and participates in cellular nutrient metabolism.
Biomimetics of nicotinamide adenine dinucleotide (mNADH) are promising cost-effective alternatives to their natural counterpart for biosynthetic applications; however, attempts to recycle mNADH often rely on coenzymes or precious metal catalysts. Direct electrolysis is an attractive approach for recycling mNADH, but electrochemical reduction of the oxidized mimetic (mNAD+) primarily results in the formation of an enzymatically inactive dimer. Herein, we find that aqueous electrochemical reduction of an NAD+mimetic, 1-n-butyl-3-carbamoylpyridinium bromide (1+), to its enzymatically active form, 1,4-dihydro-1-n-butyl nicotinamide (1H), is favored in the presence of sodium pyruvate as a supporting electrolyte. Maximum formation of 1H is achieved in the presence of a large excess of pyruvate in combination with a large excess of a co-supporting electrolyte. Formation of 1H is found to be favored at pH 7, with an optimized product ratio of ∼50/50 dimer/1H observed by cyclic voltammetry. Furthermore, sodium pyruvate is shown to promote electroreductive generation of the 1,4-dihydro form of several additional mNADH as well as NADH itself. This method provides a general strategy for regenerating 1,4-dihydro-nicotinamide mimetics of NADH from their oxidized forms1.
Synthesis
The received pyruvic acid is left in the workshop for over an hour. The extraction tube is covered with a clean polyester fabric at the mouth, slowly inserted into the bottom of the drum. Using a vacuum, 280 kg of pyruvic acid is drawn into the sodium pyruvate reaction tank. After completion, stirring is initiated. Pre-treated 3842°C sodium carbonate solution is slowly pressurized into the reaction tank (maintaining pressure between 0.030.06 MPa), controlling the reaction tank temperature below 35°C. If the temperature exceeds 35°C during the reaction, cooling measures should be taken. pH value is monitored during the reaction, and feeding stops when the pH reaches 4.85.3 (determined by precision paper). The feeding duration is about one hour. After completion, 1440 kg of 95% ethanol is slowly drawn in, with the mouth covered with a clean polyester fabric (the first 50% of the quantity should be added slowly within 60 minutes, and the next 50% within 30 minutes). After drawing is complete, centrifuge and place in a dedicated stainless steel tank, then transfer to a double-cone vacuum dryer. Control the vacuum degree to be ≥-0.09 MPa, with hot water temperature at 7580°C. When the temperature inside the vacuum dryer rises to 65°C, record the drying time. Control the temperature inside the vacuum dryer at 65~70°C, maintain insulation for 2 hours. After 2 hours of drying, perform an inspection. Dry loss should be ≤0.5%. Cool for approximately 20 minutes, discharge, sieve through a 20-mesh screen, package, and store. The yield should be within 105±5%.
Safety
Sodium pyruvate is generally considered safe for laboratory and cell culture applications, provided that standard safety precautions are followed. It is important to wear appropriate personal protective equipment, including gloves and safety goggles, to prevent skin contact and eye exposure. Avoid inhaling sodium pyruvate dust or mist, and in case of accidental ingestion, seek immediate medical attention. If skin contact occurs, wash the affected area thoroughly with water, and in case of eye contact, rinse the eyes with plenty of water and seek medical attention if irritation persists. Store sodium pyruvate in a cool, dry place away from incompatible substances, following supplier recommendations. Be aware of chemical compatibility, and in the event of a spill, follow established spill response procedures. Dispose of waste in accordance with local regulations, and consult the material safety data sheet (MSDS) for detailed safety information. Adhering to these guidelines and local safety regulations ensures the safe handling of sodium pyruvate in the laboratory2.
Reference
1. Bruggeman C, Gregurash K, Hickey DP. Impact of sodium pyruvate on the electrochemical reduction of NAD+ biomimetics. Faraday Discuss. 2023 Oct 31;247(0):87-100.
2. Slovin PN, Huang CJ, Cade JR, Wood CE, Nasiroglu O, Privette M, Orbach P, Skimming JW. Sodium pyruvate is better than sodium chloride as a resuscitation solution in a rodent model of profound hemorrhagic shock. Resuscitation. 2001 Jul;50(1):109-15.
Related articles And Qustion
See also
Lastest Price from Sodium pyruvate manufacturers
US $0.00/kg2025-09-18
- CAS:
- 113-24-6
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kgs

US $0.00/KG2025-04-21
- CAS:
- 113-24-6
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month