Selexipag: uses, Chemical properties, and Pharmacokinetics
Introduction
Selexipag (Uptravi®), approved in the US in 2015, is an orally available selective non-prostacyclin agonist to the prostacyclin IP receptor. Selexipag is hydrolyzed to an active metabolite, approximately 37-fold more potent than selexipag. Both selexipag and its metabolite are highly selective for the IP receptor, and the metabolite has a 7.9-h half-life, which allows for twice daily dosing. Common side effects include headache, diarrhea, jaw pain, nausea, myalgia, vomiting, pain in extremity, and flushing.
Uses
Selexipag is used in adults to treat pulmonary arterial hypertension (PAH, high blood pressure in the vessels that carry blood to the lungs) to slow down the worsening of symptoms and reduce the chance of being hospitalized for PAH.
Chemical property

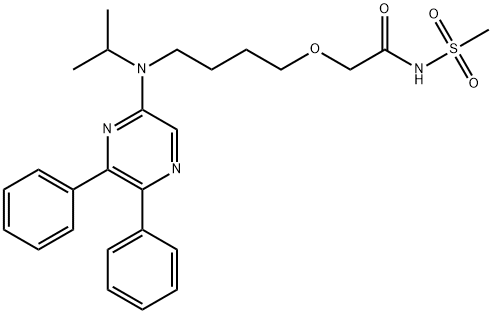
Selexipag is a pale yellow crystalline powder that is practically insoluble in water. The chemical name of this drug is 2-{4-[(5,6-diphenylpyrazin-2-yl)(isopropyl)amino]butoxy}-N (methylsulfonyl) acetamide. It has a molecular formula of C26H32N4O4S and a molecular weight of 496.62. Selexipag is very stable, not hygroscopic, and not light sensitive in the solid state. Depending on the dose strength, each round film-coated tablet for oral administration contains 200, 400, 600, 800, 1000, 1200, 1400, or 1600 mcg of selexipag. The tablets include the following inactive ingredients: D-mannitol, corn starch, low substituted hydroxypropylcellulose, hydroxypropylcellulose, and magnesium stearate. The tablets are film coated with a coating material containing hypromellose, propylene glycol, titanium dioxide, and carnauba wax, along with mixtures of iron oxide red, iron oxide yellow or iron oxide black.
Mechanism of Action
Selexipag is an oral prostacyclin receptor (IP receptor) agonist structurally distinct from prostacyclin. Selexipag is hydrolyzed by carboxylesterase 1 to yield its active metabolite, which is approximately 37-fold as potent as selexipag. Selexipag and the active metabolite are selective for the IP receptor versus other prostanoid receptors (EP1-4, DP, FP and TP).
Pharmacokinetics
The safety, tolerability, and Pharmacokinetics of selexipag were initially evaluated in several dose-ranging studies on healthy, normal volunteers. Selexipag has a half-life of 0.8–2.5 hours and is hydrolyzed to its pharmacologically active metabolite ACT-333679 with a half-life of 6.2–13.5 hours. It is rapidly absorbed after oral administration and may be taken with or without food. Single doses above 400 μg led to an increased incidence of headache, nausea, dizziness, and vomiting in normal volunteers. Gradual up-titration improves tolerability, and the maximum tolerated strength with repeated dosing in normal volunteers was 1,600 μg. In the presence of food, the absorption of selexipag was prolonged, resulting in a delayed time to peak concentration (Tmax) and approximately 30% lower peak plasma concentration (Cmax). The exposure to selexipag and the active metabolite (area under the curve [AUC]) did not significantly change in the presence of food.
Steady-state is achieved within 3 days of twice daily dosing. Elimination is mainly via the hepatobiliary route, and renal and hepatic impairment increases exposure to selexipag and its metabolite ACT-333679. Greater caution is recommended during up-titration in individuals with liver or renal impairment, and once-daily administration is recommended for patients with moderate liver impairment. Use of selexipag should be avoided in those with severe hepatic impairment. The initiation of selexipag in patients with impaired renal function is similar to that of patients with normal renal function. However, caution during titration is advised. There is a 40%–70% increase in exposure to selexipag and its active metabolite in patients with estimated glomerular filtration rates of 15–30 mL/minute/1.73 m2. Of note, there have been no studies involving patients with severely impaired renal function (estimated glomerular filtration rate <15 mL/minute/1.73 m2).
References:
[1] MARTHA KINGMAN. Management of prostacyclin side effects in adult patients with pulmonary arterial hypertension.[J]. Pulmonary Circulation, 2017, 7 3. DOI:10.1177/2045893217719250.You may like
See also
Lastest Price from Selexipag manufacturers
US $0.00-0.00/kg2025-08-22
- CAS:
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1

US $0.00/KG2025-04-21
- CAS:
- 475086-01-2
- Min. Order:
- 10g
- Purity:
- 99%min
- Supply Ability:
- 10kg