Safety Evaluation of Tocopheryl acetate and its derivatives
Introduction of Tocopheryl acetate
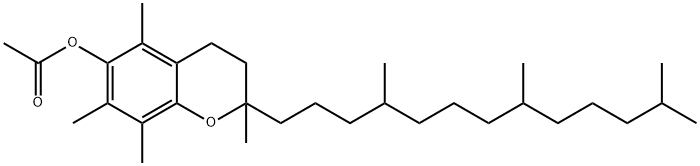
Tocopheryl acetate is the synthetic form of vitamin E and the best choice for the treatment of vitamin E deficiency. It has antioxidant properties and is thought to protect cells from free radicals. It is primarily used as a dietary supplement, ingredient in skin care formulations and in animal feed. In nature, vitamin E comes in the form of tocopheryl or tocotrienol. Both tocopheryl and tocotrienol have four forms, known as alpha, beta, gamma, and delta. Alpha-tocopheryl (AT) is the most active form of vitamin E in humans.

Tocopherol and its several ester and ether derivatives all function as antioxidants in cosmetic formulations; they also have other functions, such as skin conditioning. Tocopheryl Acetate, Tocopherol, and Tocopheryl Linoleate are used in 2673 formulations, generally at concentrations of up to 36%, 5%, and 2%, respectively, although Tocopheryl Acetate is 100% of vitamin E oil. Tocophersolan, Tocopheryl Linoleate/Oleate, Tocopheryl Nicotinate, Tocopheryl Succinate, Dioleyl Tocopheryl Methylsilanol, and Potassium Ascorbyl Tocopheryl Phosphate, combined, are used in 36 formulations at concentrations lower than those reported for the frequently used ingredients. Tocopherol may be isolated from vegetable oils or synthesized using isophytol and methylhydroquinone. Tocopherol, Tocopheryl Acetate, Tocopheryl Linoleate, and Tocopheryl Succinate all were absorbed in human skin. In rat skin, Tocopheryl Acetate is hydrolyzed to Tocopherol. Tocopherol is a natural component of cell membranes thought to protect against oxidative damage. Tocopherol, Tocopheryl Acetate, and Tocopheryl Succinate each were reported to protect against ultraviolet radiation-induced skin damage.
Safety Evaluation
These ingredients are generally not toxic in animal feeding studies, although very high doses (>2 g/kg/day) have hemorrhagic activity. These ingredients are generally not irritating or sensitizing to skin or irritating to eyes, although a Tocopheryl Acetate did produce sensitization in one animal test, and Tocophersolan was a slight eye irritant in an animal test. Reproductive and developmental toxicity tests in animals using Tocopherol, Tocopheryl Acetate, Tocopheryl Succinate, and Tocophersolan were all negative or showed some effect of reducing toxicity. Tocopherol, Tocopheryl Acetate, Tocopheryl Succinate, and Dioleyl Tocopheryl Methylsilanol were almost uniformly negative. These ingredients exhibit antimutagenic activity consistent with their antioxidant properties.
Tocopherol was not carcinogenic. The ability of Tocopherol, Tocopheryl Acetate, and Tocopheryl Succinate to modulate the carcinogenic effect of other agents (e.g., tumor promotion) has been extensively studied. One study showing tumor promotion in mice may be discounted as not reproducible and not consistent with the large volume of data suggesting that the antioxidant properties of these agents protect against tumor induction. Specifically, the frequent use of Tocopherol as a negative control in other tumor promotion studies suggests that Tocopherol is not a tumor promoter. Tocopherol has been shown to reduce the photocarcinogenic effect of ultraviolet radiation in mice. Similar studies with Tocopheryl Acetate and Tocopheryl Succinate, however, demonstrated some enhancement of photocarcinogenesis, although the effect was not dose related. In clinical studies, Tocopherol, Tocopheryl Acetate, and Tocopheryl Nicotinate were not irritants or sensitizers. A report of a large number of positive patch-tests to Tocopheryl Linoleate in one cosmetic product were considered to result from a contaminant or metabolite.
The Cosmetic Ingredient Review Expert Panel considered that these data provide an adequate basis on which to conclude that Tocopherol, Tocophersolan, Tocopheryl Acetate, Tocopheryl Linoleate, Tocopheryl Linoleate/Oleate, Tocopheryl Nicotinate, Tocopheryl Succinate, Dioleyl Tocopheryl Methylsilanol, and Potassium Ascorbyl Tocopheryl Phosphate are safe as used in cosmetic formulations. Although there were no inhalation toxicity data, these ingredients are used at such low concentrations in hair sprays that no inhalation toxicity risk was considered likely. Because methylhydroquinone is used in the chemical synthesis of Tocopherol, there was concern that hydroquinone may be present as an impurity. In such cases, residual levels of hydroquinone would be expected to be limited to those achieved by good manufacturing practices.
References:
[1] FIUME M Z. Final report on the safety assessment of Tocopherol, Tocopheryl Acetate, Tocopheryl Linoleate, Tocopheryl Linoleate/Oleate, Tocopheryl Nicotinate, Tocopheryl Succinate, Dioleyl Tocopheryl Methylsilanol, Potassium Ascorbyl Tocopheryl Phosphate, and Tocophersolan.[J]. International Journal of Toxicology, 2002. DOI:10.1080/10915810290169819.
You may like
See also
Lastest Price from Tocopheryl acetate manufacturers

US $0.00/kg2025-09-03
- CAS:
- 7695-91-2
- Min. Order:
- 20kg
- Purity:
- 98%; EP/USP/FCC
- Supply Ability:
- 1000KGS

US $0.00-0.00/KG2025-08-30
- CAS:
- 7695-91-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 2000KG