Reactions of Trifluoromethanesulfonamide and its derivatives
Description
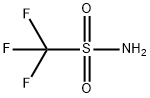
Several trifluoromethanesulfonamide derivatives are used as drugs in medicine or as pesticides, insecticides, fungicides, etc., in agriculture. Trifluoromethanesulfonamide (from now on, “triflamide” for brevity) and its various N-substituted derivatives represent the most numerous class of triflic compounds[1].
1. N-Halogenation Reactions.
Trifluoromethanesulfonamide is fluorinated with fluorine/nitrogen gas mixture in the presence of NaF/KF fusion at −65 °C to give N, N-difluorotriflamide in 33% yield.56 The product is a pale-brown gas, explosive on impact or heating; below 10 °C, it is condensed into a colorless liquid.
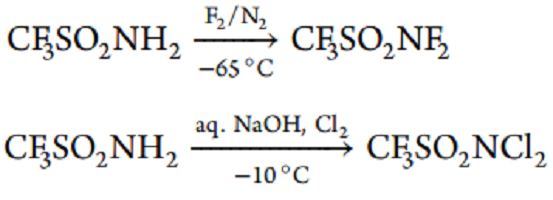
In the same work, N, N-dichlorotriflamide was first synthesized in 55% yield by saturation of the alkaline solution of triflamide with chlorine upon cooling.
2. N-Alkylation reactions.
N-alkylation of triflamide, that is, the formation of new N−C bond, is an alternative route to numerous N-substituted triflamides, which, because of their high acidity and lipophilicity, imparted by the trifluoromethyl sulfonyl group, is inherent to a large number of these compounds. The reaction proceeds via the intermediate triflamide anion generated by alkali metal carbonates, as exemplified below
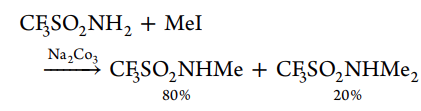
Several monoalkylated derivatives were prepared by the K2CO3-catalyzed alkylation of the primary perfluoroalkyl sulfonyl amides with alkyl halogenides or tosylates.
3. Condensation Reactions of Triflamide.
A new type of reaction of triflamide was discovered when studying transformations occurring in the system triflamide−paraformaldehyde under strongly acidic conditions. Strange as it may seem, despite the well-known ability of sulfonamides to enter condensation reactions with carbonyl compounds, no such reactions were observed until recently. A possible reason is that the basicity of nitrogen in triflamide is lower than in other sulfonamides, and it does not react even with highly electrophilic chloral molecules, which gives adducts with sulfonamides without special activation.
4. Miscellaneous Reactions of Triflamides.
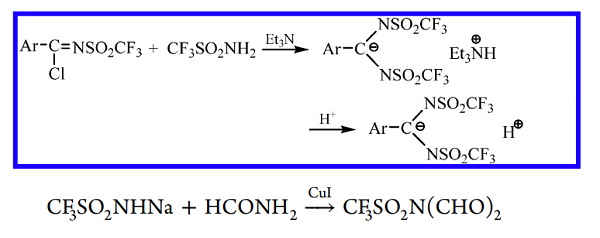
N-(Triflyl)carboxyimidoyl chlorides Ar−C(Cl)=NSO2CF3, isoelectronic to aroyl chlorides, react with triflamide in the presence of triethylamine to give salts, which, after acidification give N, N-bis(triflyl)benzamidines. The product has the ionic structure as depicted below because of the superstrong electron-withdrawing ability of the triflamide residue CF3SO2N= (vide infra). The first N-formyl triflamide was prepared by the CuI-catalyzed reaction of the sodium salt of triflamide with formamide.
References
[1] Bagrat A. Shainyan*, Ljudmila L. Tolstikova. “Trifluoromethanesulfonamides and Related Compounds.” Chemical Reviews 113 1 (2012): 699–733.
You may like
See also
Lastest Price from Trifluoromethanesulfonamide manufacturers

US $0.00-0.00/KG2025-12-10
- CAS:
- 421-85-2
- Min. Order:
- 1KG
- Purity:
- 98.5% min
- Supply Ability:
- 2 tons/year

US $10.00/KG2025-04-21
- CAS:
- 421-85-2
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt