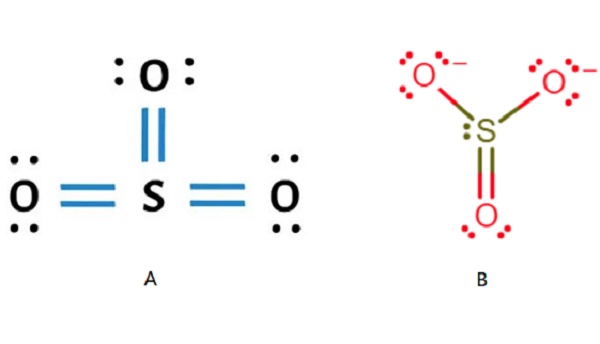Polarity of Sulfur Trioxide (SO3)
Introduction of Sulfur Trioxide (SO3)
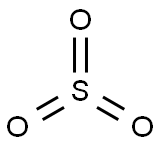
Sulfur trioxide (SO3) consists of a central sulfur (S) atom surrounded by three oxygen (O) atoms. Sulfur and oxygen have six valence electrons each. Sulfur forms double covalent bonds with each oxygen atom. As a result, the SO3 structure is trigonal planar, with a bond angle of 120°. No lone pairs on the central sulfur atom make the molecule symmetric.
Molecular polarity determination
The polarity of a molecule can be determined by composition, geometry, dipole moment and bond formation. If the dipole moment of a molecule is zero, it means that the molecule is non-polar. Being non-polar, the molecule has equal charge distribution. If the atoms involved in a molecule have equal charge distributions, it has a net dipole moment of zero, while molecules with net dipole moments have unequal charge distributions because they are inherently polar. The dipole moment of a molecule is calculated as the product of the bond length charges on each element.
Polarity of Sulfur Trioxide (SO3)
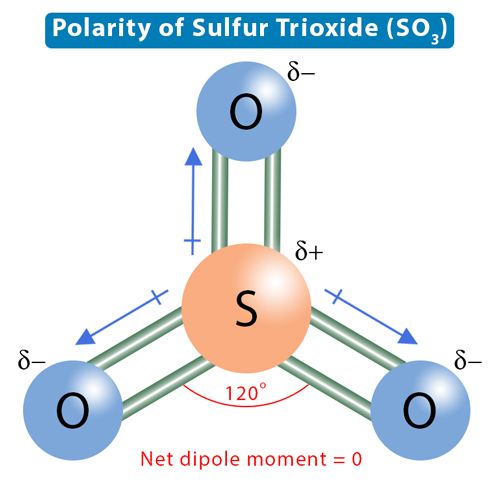
The electronegativities of sulfur and oxygen are 2.58 and 3.44, respectively. Sulfur is the least electronegative, which explains why it is at the molecule's center. Oxygen is the more electronegative atom, and it will attract the shared electron pairs toward itself. It will acquire a partial negative charge, and sulfur will acquire a partial positive charge. Therefore, the two atoms' electronegativity difference makes the S-O bond polar. Each S-O bond will have a dipole moment directed from sulfur to oxygen. However, due to the symmetry of the trigonal planar structure, these dipole moments will cancel, and the net dipole moment will be zero. Therefore, sulfur trioxide is nonpolar.
Properties of Sulfur trioxide (SO3)
SO3 is a strong oxidising agent, using a wide range of molecular species and crystalline forms. Because sulphur has a +6 oxidation state, this is the highest compared to the other atoms in the molecule. In the liquid state it is colourless and odourless and in the solid state it can be seen as a crystalline form. In the gaseous state, it acts as a pollutant and can be examined by using acid rain as an example. Rainwater mixed with SO3 is acid rain and is harmful to aquatic life and humans.