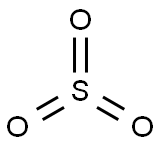
The Lewis structure of Sulfur trioxide
Description
Sulfur trioxide is a compound with the chemical formula SO3. This compound is widely postulated as the active sulfonating agent in electrophilic aromatic substitutions. Sulfur trioxide is a crucial compound for atmospheric sulfuric acid (H2SO4) formation, acid rain formation, and other atmospheric physicochemical processes. During the daytime, SO3 is mainly produced from the photo-oxidation of SO2 by OH radicals. However, the sources of SO3 during the early morning and night, when OH radicals are scarce, still need to be fully understood. Sulfur trioxide is not only environmentally harmful but also highly corrosive and poses a significant threat to the safe operation of coal-fired power plants[1-2].
Sulfur trioxide readily acts as a Lewis acid to form addition compounds with many substances, but boron trifluoride is a much stronger Lewis acid than sulfur trioxide. Douglas et al. demonstrated the unusually high Lewis acidity of SO3, which forms an adduct with the Lewis base NH3, more potent than that found with the analogous Lewis acid SO2[3].
The Lewis structure of SO3
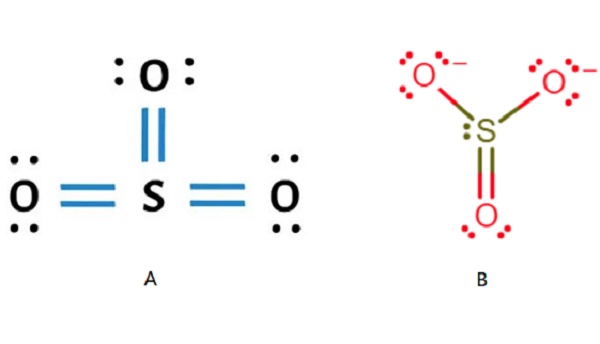
Theoretically, the Lewis structure of SO3 is shown in the figure (B) above. But because these three bonds are conjugated, they are actually completely equivalent. Therefore, people often use the above figure (A) to represent the Lewis structure of so3
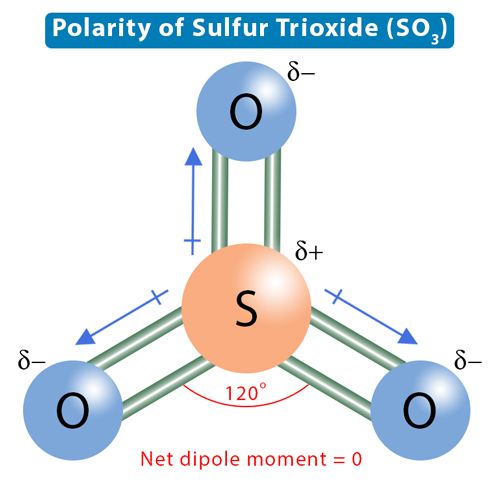
Lewis structure of SO3 (or Sulfur trioxide) contains three double bonds between the Sulfur (S) atom and each Oxygen (O) atom. The Sulfur atom (S) is at the center, surrounded by 3 Oxygen atoms (O). The Sulfur atom does not have a lone pair, while all three Oxygen atoms have 2 lone pairs. The bond angle in the SO3 molecule is approximately 120 degrees. This is due to the trigonal planar molecular geometry, where the three oxygen atoms are arranged symmetrically around the central sulfur atom.
What is Lewis acid?
In the Lewis definitions ofacids and bases, a Lewis acid is an electron pair 'acceptor,' which will acquire an electron pair. Similar to BF3, which is isoelectronic, SO3 has an antibonding MO, formed from the sulfur 3pz and oxygen 2pz atomic orbitals, that can accept electrons, thereby exhibiting Lewis acid behavior, which is the definition of an electrophile.
References
[1] Dai, Gaofeng, et al. "Catalytic function of ferric oxide and effect of water on the formation of sulfur trioxide." Journal of Environmental Management 264(2020):110499.
[2] Yao, Lei, et al. "Unprecedented Ambient Sulfur Trioxide (SO 3 ) Detection: Possible Formation Mechanism and Atmospheric Implications." (2020).
[3] Douglas, John E. , G. L. Kenyon , and P. A. Kollman . "The ammonia—sulfur trioxide interaction. An ab initio study." Chemical Physics Letters 57.4(1978):553-556.