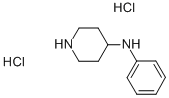
N-phenylpiperidin-4-amine dihydrochloride- reaction / application on synthetic works
N-phenylpiperidin-4-amine dihydrochloride is an important organic intermediate to synthetize substituted phenylpiperidin products.
Application
The following example is about its application on the synthesis of cinnoline derivatives useful as cb-1 receptor inverse agonists [1]
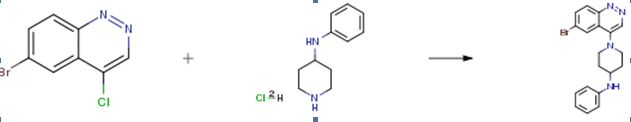
Into a 250-mL round-bottom flask, was placed tert-butyl 4- (phenylamino)piperidine-l -carboxylate (14 g, 50.66 mmol, 1.00 equiv) and sat. HCI/EtOH (30 mL). The resulting solution was stirred overnight at room temperature. The resulting mixture was concentrated under vacuum. The resulting residue was dissolved in ethyl acetate (100 mL).
The solids were collected by filtration and washed with ethyl acetate (50 mL) to yield N- phenylpiperidin-4-amine dihydrochloride as a white solid. Into a 100-mL round-bottom flask, was placed a solution of 6-bromo-4- chlorocinnoline (300 mg, 1 .23 mmol, 1 .00 equiv) in isopropanol (30 mL), N- phenylpiperidin-4-amine dihydrochloride (1 .23 g, 4.94 mmol, 4.00 equiv) and DIEA (2.14 mL, 10.00 equiv). The resulting solution was stirred overnight at 90°C. The resulting mixture was concentrated under vacuum. The resulting residue was dissolved in sat. sodium bicarbonate (50 mL). The resulting solution was extracted with ethyl acetate (2×100 mL) and the organic layers combined and dried over sodium sulfate. The solids were filtered out. The resulting mixture was concentrated under vacuum. The resulting residue was applied onto a silica gel column with ethyl acetate to yield 1 -(6-bromocinnolin-4- yl)-N-phenylpipehdin-4-amine as a yellow solid.
The following example is about its application on the synthesis of aminohaloborane [2]
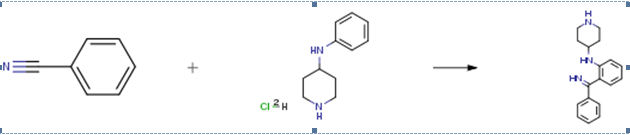
To a stirred solution of BCl3 (25×12 mmol) in toluene (15 ml) was added a solution of a N-monoaminoalkylaniline (25mmol) in toluene (40 ml) under ice cooling. The resulting mixture was refluxed for 1 h and then the solvent was distilled off. To the resulting syrup, a nitrile (25×2 mmol) was added, and the mixture was heated at 150°C for 3 h under stirring. The progress of the reaction was monited by TLC, which showed a yellow sport. After cooling, ice and 1N HCl (15 ml) were added and the mixture was warmed at 100°C for 20 min under stirring a hydrolyze the corresponding ketimine. The hydrochloride of the compound crystallized, it was filtered off and recrystallized.
References
Janssen Pharmaceutica Nv. Zhang YM, Decorte BL, Greco MN. Ludovici DW, Parker MH. Macielag MJ. Cinnoline Derivatives Useful As Cb-1 Receptor Inverse Agonists, WO2016/115013[P], 2016, A1, Page column 87; 88
Adachi M, Sasakura K, Sugasawa T. Aminohaloborane in Organic Synthesis. IX. Exclusive ortho Acylation Reaction of N-Monoaminoalkylanilines[J], Chemical and pharmaceutical bulletin, 1985, 33(5):1826-1835.