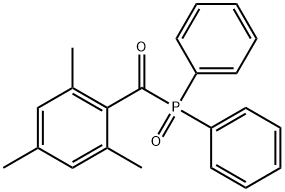
Diphenyl(2,4,6-trimethylbenzoyl)phosphine oxide - reaction / application on synthetic works
Diphenyl(2,4,6-trimethylbenzoyl)phosphine oxide is an organic photoinitiator. It is also an important organic intermediate to synthetize polymerization initiators in the area of materials science, miscellaneous, and polymer science.
The following example is about its application on the synthesis of the acylphosphine oxide -4 -hydroxyphenylsulfonium salt compound [1]
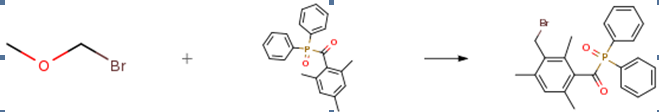
2,4,6-trimethylbenzoyldiphenylphosphine oxide (TPO) (0.1 mol) was dissolved in 100 mL of anhydrous dichloromethane. Add anhydrous zinc dibromide (0.15 mol) in portions and stir. The dichloromethane solution (0.12 mol) of bromodimethyl ether was added dropwise at 10 ° C, and the reaction was completed after 0.5 h, and the reaction was stirred at room temperature for 6 h. After monitoring the reaction, the plate was slowly added to an equal volume of ice water with a solvent, and the organic layer was washed with deionized water and dried over anhydrous sodium sulfate. The product was subjected to ethyl acetate/cyclohexane silica gel column chromatography to give the product. Yield 87%, pale yellow solid.
The following example is about its application on the synthesis of water-soluble photopolymerization initiator [2]

After 133 parts of dichloromethane (CH2Cl2) was dissolved in 2,4,6-trimethylbenzoyldiphenylphosphine oxide (30.0 parts, 86.1 molar equivalents, IRGACURE TPO manufactured by BASF), anhydrous aluminum(III) chloride (AlCl3, 45.9 parts, 344.5 molar equivalents) was added thereto in portions and dissolved by performing stirring. Then, chloroacetyl chloride (57.9 parts, 516.7 molar equivalents) was added dropwise and stirred at room temperature (rt, 10° C. to 35° C, the same applies hereafter) for 7 hours. The reaction solution was gradually added dropwise to ice water to stop the reaction, then extracted with ethyl acetate, washed with water, and dried with magnesium sulfate. The resulting filtrate was concentrated, purified using a silica gel column (hexane/ethyl acetate=50/50 (volume ratio)), concentrated, and then washed with hexane to obtain a white compound (33.65 parts, yield 92percent).
The following example is about its application on the synthesis of Thio- or Selenophosphinates [3]

TMDPO (278.7 mg, 0.8 mmol), alkyl iodide 11 (0.2 mmol), 1,1,1,2,2,2-hexabutyldistannane (0.2 mmol), and dry, degassed toluene (0.6 mL, Scheme 10 a) or BTF [(1′,1′,1′-trifluoromethyl)benzene] (0.6 mL, Scheme 10 b] were placed in a sealed Pyrex NMR tube under an inert atmosphere, and the mixture was irradiated with a xenon lamp (500 W) for 6 h at r.t. Elemental sulfur (1 mmol) was added under an inert atmosphere, and the mixture was stirred for 6 h at 40 °C. The reaction mixture was concentrated, and product was isolated by gel permeation chromatography.
References
Tongji University. Jin M, Wan D, Pan H. Acylphosphine oxide -4 -hydroxyphenylsulfonium salt compound as well as preparation method and application thereof (by machine translation), CN110156835[P], 2019, A, Paragraph 0064-0066
Fujifilm Corporation. Yokoi K, Tsuyama H. Aqueous curable composition and water-soluble photopolymerization initiator. US2018/362558[P], 2018, A1, Paragraph 0316-0317
Sato Y, Kawaguchi SI, Nomoto A, Ogawa A. Photoinduced Coupling Reaction of Diphenyl(2,4,6-trimethylbenzoyl)phosphine Oxide with Interelement Compounds: Application to the Synthesis of Thio- or Selenophosphinates. Synthesis, 2017, 49(16):3558 - 3567
You may like
Related articles And Qustion
Lastest Price from Diphenyl(2,4,6-trimethylbenzoyl)phosphine oxide manufacturers
US $0.00-0.00/KG2025-12-02
- CAS:
- 75980-60-8
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $1.10/g2025-09-15
- CAS:
- 75980-60-8
- Min. Order:
- 1g
- Purity:
- 99.9%
- Supply Ability:
- 100 Tons Min