Methanesulfonic anhydride:a protective reagent
Introduction
Methanesulfonic anhydride(also methylsulfonyl anhydride), a widely employed methanesulfonylation reagent, holds significant importance in organic synthesis due to its versatile applications. This compound is frequently utilized in reactions involving bases such as pyridine, 2,4,6-trimethylpyridine, or triethylamine, wherein it exhibits reactivity with alcohols to yield the corresponding methylsulfonyl esters. Notably, in comparison to the commonly used methanesulfonylation reagent, methanesulfonyl chloride, the use of methylsulfonyl anhydride offers the advantage of preventing the formation of chlorinated by-products during the reaction.
Application
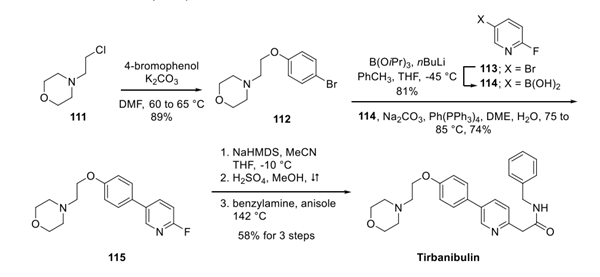
Methanesulfonic anhydride is a commonly used methanesulfonylation reagent. One of its most common applications is in the presence of bases such as pyridine, 2,4,6-trimethylpyridine, or triethylamine, where it reacts with alcohols to produce the corresponding methylsulfonyl esters (see Equation 1)1. Compared to another widely used methanesulfonylation reagent, methanesulfonyl chloride, the use of this reagent can prevent the generation of chlorinated by-products during the reaction. However, this reagent may not yield satisfactory results in reactions with unsaturated alcohols or may lead to the formation of other products (see Equation 2)2. This reagent reacts with amines to produce methanesulfonamide, which is a commonly used method for protecting amine (amino) groups. Under acidic and alkaline conditions, methanesulfonamide can exist stably. Using LiAlH4 can deprotect methanesulfonamide and obtain free amines again. Meanwhile,under the catalysis of Lewis acid, the reagent can undergo Friedel Crafts sulfonation reaction with benzene or substituted benzene. When using methanesulfonic anhydride as a sulfonation reagent, only benzene can undergo this reaction. When using this reagent, substituted benzene compounds can also achieve good yields. By adding sulfuric acid to the system, diarylsulfone can be obtained, and electron donating substituents on the aromatic ring will greatly increase the yield of the reaction. In addition to the common reactions mentioned above, this reagent can be used in combination with dimethyl sulfoxide to oxidize alcohols. using methanesulfonic anhydride as the solvent, the combination reagent generated by this reagent and dimethyl sulfoxide can oxidize primary alcohols to corresponding aldehydes; By using secondary alcohols as raw materials, corresponding ketones can be obtained.
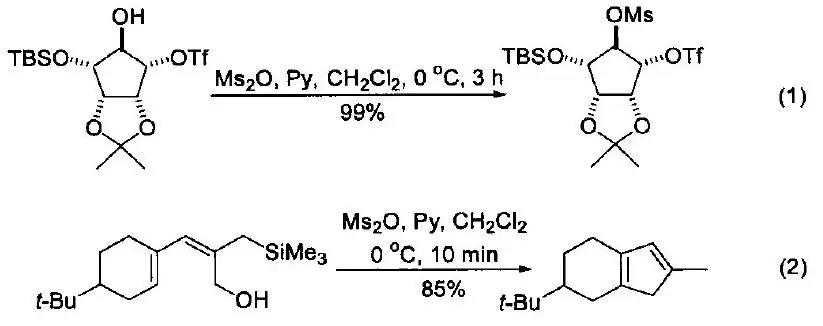
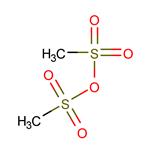
Figure 1 Commonly used protective reagent:Methanesulfonic anhydride
Synthesis
The experimental procedure for synthesizing methanesulfonic anhydride involves mixing 300 grams of phosphorus pentoxide with 90 grams of diatomaceous earth. Half of this mixture is taken, and under stirring, 305 grams of methylsulfonic acid and 10 grams of asbestos are added. The reaction is heated at 80°C for 2.5 hours, and when the reaction rate decreases, the remaining half of the phosphorus pentoxide and diatomaceous earth is gradually introduced, followed by an additional 3 hours of heating. The reaction mixture is then extracted with dichloromethane, and the solvent is evaporated. The resulting mixture undergoes vacuum distillation, with the fraction collected at 105-109°C (0.133 kPa). The crude product obtained is 193 grams, which is purified through recrystallization with ethyl ether-benzene, yielding 143 grams of methylsulfonyl anhydride. The overall yield of the process is 55%. It is important to note that safety precautions must be followed due to the involvement of toxic substances in the experiment. It is corrosive and sensitive to moisture, and should be stored in a cool and dry place.
Safety
It is imperative to emphasize that methanesulfonic anhydride possesses certain chemical properties that necessitate careful handling and storage. Not only is it corrosive, posing potential risks to skin, eyes, and mucous membranes, but it is also sensitive to moisture. As a hygroscopic substance, exposure to water can lead to degradation, compromising its efficacy and safety. Therefore, meticulous storage conditions are paramount to ensure the stability of the compound. To safeguard the integrity of methylsulfonyl anhydride, it is recommended to store it in a cool and dry environment. The storage facility should be adequately ventilated to prevent the accumulation of vapors and maintain optimal conditions for the compound. Furthermore, the storage containers must be tightly sealed to prevent moisture ingress and subsequent degradation. Researchers and practitioners working with methylsulfonyl anhydride should adhere to stringent safety protocols. This includes wearing appropriate personal protective equipment (PPE) such as gloves and goggles, conducting reactions in well-ventilated areas or under fume hoods, and exercising caution to prevent accidental skin or eye contact. In conclusion, while methylsulfonyl anhydride serves as a valuable reagent in synthetic chemistry, its corrosive nature and sensitivity to moisture underscore the importance of diligent storage practices and adherence to safety measures in laboratory settings.
Reference
1. Hu,G.; VaseUa, A. Heh. Chim. Acta 2004,87,2405.
2. Kuroda, C.; Okada, M.; Shinozaki, S.; Suzuki, H. Tetrahedron 2006, 62,726.
You may like
Related articles And Qustion
See also
Lastest Price from Methanesulfonic anhydride manufacturers
US $0.00-0.00/kg2025-11-20
- CAS:
- 7143-01-3
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 100tons
US $10.00/kg2025-04-21
- CAS:
- 7143-01-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100 mt