Tirbanibulin: Synthesis and Introduction
Introduction of Tirbanibulin
Tirbanibulin, a first-in-class dual inhibitor of tyrosine-protein kinase CSK, more commonly termed Src kinase, was developed by Athenex Inc. (formerly Kinex Pharmaceuticals) and approved by the USFDA in December 2020 for the topical treatment of actinic keratosis of the face orscalp. In Phase 3 clinical trials, the drug achieved complete reduction of lesions within two months of treatment with dramatically less toxicity than standard topical therapies for actinic keratosis. An account of the discovery of a potent, nonpeptide, non-ATP competitive Src inhibitor, culminating in the identification of tirbanibulin, has been detailed in the chemical literature.
Synthesis of Tirbanibulin
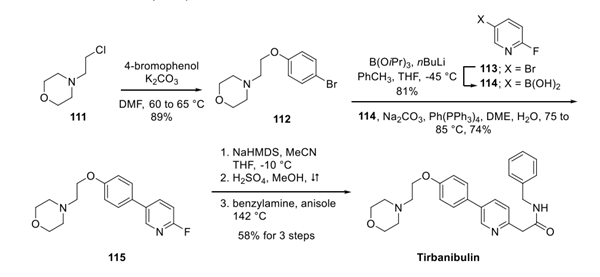
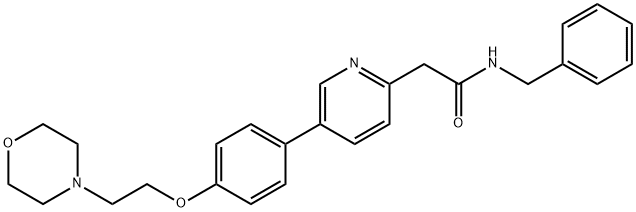
Alkylation of chloride 111 with 4-bromophenol gave ether 112. Suzuki coupling with pyridyl boronic acid 114 (which arose from the corresponding bromide 113 via a Miyaura coupling) generated ether 115. Substitution of the fluoropyridine with deprotonated acetonitrile was followed by subsequent treatment with acid and benzylamine in anisole at elevated temperatures to furnish tirbanibulin in 58% yield across the three step sequence.