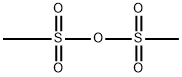
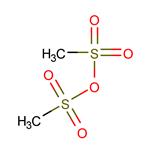
Methanesulfonic Anhydride: A Comprehensive Overview
Methanesulfonic anhydride is a off-white solid with a melting point of 71°C and a boiling point of 138°C (1.33 kPa). It is soluble in chloroform, benzene, and hot ether.

Properties
Reaction of Methanesulfonic Anhydride with Hydrogen Chloride.-Passage of anhydrous hydrogen chloride through stirred molten methanesulfonic anhydride' for periods of about 2 hours first at 55-68℃ and then at 75-80℃ did not effect complete reaction, and a recrystallized sample of the solid which formed on cooling had m.p. 63-65.5℃, unde- pressed by the original anhydride. After continued pas- sage for 3.5 hours at 105℃, solidification no longer occurred on cooling, but distillation yielded anhydride (16% recovery) as well as methanesulfonyl chloride (36%, TPD 1.4496. Anal. Calcd. for CH3ClO2S: C,10.49; H, 2.64. Found: C, 10.84; H, 2.90).
Reactions
More vigorous conditions were necessary in the reaction of methanesulfonic anhydride with hydro- gen bromide than had been used in the reaction of benzenesulfonic anhydride, in order to effect completeness of reaction. Methanesulfonyl bro- mide was obtained in 44% yield. No dimethyl disulfide could be isolated ; conceivably, sulfenyl halides could have been formed and overlooked in the isolation procedure, although a disulfide and not a sulfenyl halide is the principal product with benzenesulfonic anh~dride.~ Nevertheless, bro- mine vapors were evident during the reaction, and titration of the residue for methanesulfonic acid after distillation of the sulfonyl bromide showed an excess over that anticipated from equation 1, which represents simple cleavage.
Applications
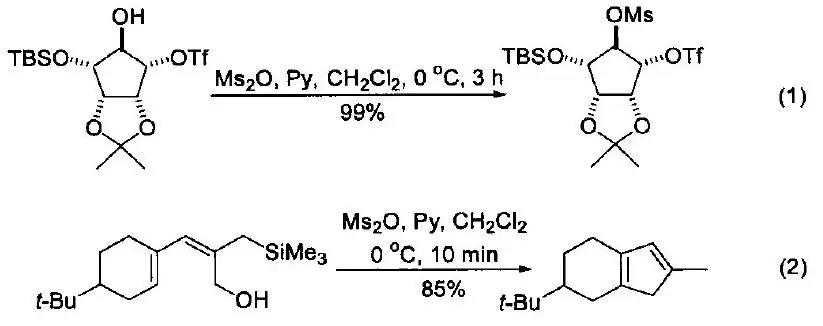
Methanesulfonic anhydride plays a pivotal role in organic synthesis, particularly as a reagent for converting alcohols to mesylates.
Storage and Precautions
Corrosive; moisture sensitive. Store in a cool, dry place.
You may like
Related articles And Qustion
Lastest Price from Methanesulfonic anhydride manufacturers
US $0.00-0.00/kg2025-11-20
- CAS:
- 7143-01-3
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 100tons
US $10.00/kg2025-04-21
- CAS:
- 7143-01-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100 mt