1,3-Butadiene: Overview, Applications in Synthetic Rubber and Biosynthesis
General Description
1,3-Butadiene, a key compound in synthetic rubber production, is vital for manufacturing automobile tires due to its unique molecular structure with alternating double bonds. It undergoes various chemical reactions, including polymerization to create elastic materials. In synthetic rubber applications, polybutadiene, derived from 1,3-butadiene, is crucial for tire treads, offering high resistance and durability. Recent advancements have enabled the biosynthesis of 1,3-butadiene from glucose using engineered metabolic pathways in microorganisms. This innovation showcases the potential for sustainable production methods in the chemical industry.
Overview
1,3-Butadiene, a vital aliphatic organic compound with the chemical formula C4H6, is a cornerstone in the realm of synthetic rubber production. Originating in Germany during World War I, its primary source was acetylene. However, during World War II, American production shifted predominantly to butenes derived from petroleum and natural gas, with a minor portion sourced from ethyl alcohol. Notably, 1,3-butadiene emerged as the predominant form, significantly contributing to the synthetic rubber industry, particularly in the manufacturing of automobile tires. As the simplest member of conjugated dienes, 1,3-butadiene showcases a distinctive molecular structure with alternating double bonds (C=C―C=C). This unique arrangement lends itself to a plethora of chemical reactions, rendering 1,3-butadiene indispensable in chemical synthesis. Catalytic processes facilitate its polymerization, leading to the formation of elastic, rubber-like materials when combined with reactive molecules like acrylonitrile or styrene. Additionally, uncatalyzed reactions with compounds such as maleic anhydride result in Diels-Alder reactions, yielding cyclohexene derivatives. Despite its chemical versatility, 1,3-butadiene remains susceptible to attacks from various reactive substances, with reactions often involving both double bonds. For instance, the addition of chlorine generates multiple dichloro-butene isomers. Overall, 1,3-butadiene's multifaceted properties and widespread industrial applications underscore its significance in modern chemical and manufacturing processes. 1
Applications in Synthetic Rubber
1,3-Butadiene, a reactive colorless gas with the chemical formula C4H6, plays a pivotal role in the production of synthetic rubber, particularly in the form of polybutadiene. This elastomer, characterized by its high resistance to abrasion, low heat buildup, and crack resistance, finds extensive application in tire treads for trucks and automobiles. Polybutadiene is synthesized through the polymerization of butadiene, facilitated by anionic or Ziegler-Natta catalysts, resulting in giant molecules or polymers. The molecular structure of polybutadiene can vary, with cis-1,4 being the predominant form. Polybutadienes can be produced with high cis content (95 to 97 percent) or with a mixed isomer composition. The properties of these polymers differ significantly, with high cis content polybutadiene exhibiting superior resilience. In tire manufacturing, butadiene rubber is often blended with natural rubber or styrene-butadiene rubber to enhance resilience and reduce rolling resistance. Beyond tires, synthetic rubber derived from 1,3-butadiene finds application in various industries, including footwear, wire and cable insulation, conveyor belts, and the production of high-impact polystyrene and acrylonitrile-butadiene-styrene copolymer. The quest for synthetic rubber dates back to the early 20th century, with significant advancements made by chemists such as Ivan Kondakov, Sergey Lebedev, and G. Ebert. However, it was not until 1961 when Phillips Petroleum Company successfully produced poly(1,3-Butadiene), revolutionizing the synthetic rubber industry with its exceptional resilience and abrasion resistance, making 1,3-butadiene rubber the second most produced synthetic rubber after styrene-butadiene rubber. 2
Biosynthesis
1,3-Butadiene, a crucial monomer for synthetic rubber and engineering plastics, cannot be directly biosynthesized by microorganisms using glucose as a carbon source. However, in a recent study, an artificial metabolic pathway was engineered in Escherichia coli to produce 1,3-butadiene from glucose. This pathway combines the cis,cis-muconic acid (ccMA)-producing pathway with tailored mutations in ferulic acid decarboxylase. By redesigning the substrate-binding site of the enzyme through computational simulations, the decarboxylation of ccMA is enhanced, leading to increased 1,3-butadiene production. Additionally, the study highlights the significance of controlling dissolved oxygen levels and pH for optimal 1,3-butadiene yield. Utilizing a DO–stat fed-batch fermentation approach, the researchers achieved a production level of 2.13 ± 0.17 g L−1 of 1,3-butadiene. These findings demonstrate the feasibility of producing industrially important compounds like 1,3-butadiene from renewable carbon sources through rational enzyme design strategies, paving the way for sustainable manufacturing processes. 3
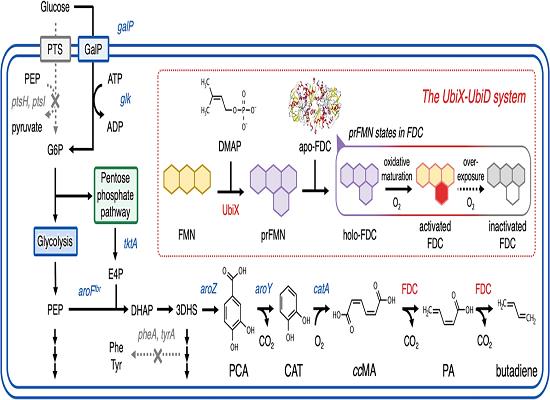
Figure 1. Biosynthesis of 1,3-Butadiene
Reference
1. Britannica, The Editors of Encyclopaedia. "butadiene". Encyclopedia Britannica. 2024.
2. Britannica, The Editors of Encyclopaedia. "butadiene rubber". Encyclopedia Britannica. 2024.
3. Mori Y, Noda S, Shirai T, Kondo A. Direct 1,3-butadiene biosynthesis in Escherichia coli via a tailored ferulic acid decarboxylase mutant. Nat Commun. 2021; 12(1): 2195.
Related articles And Qustion
Lastest Price from 1,3-Butadiene manufacturers

US $1.00/KG2025-04-21
- CAS:
- 106-99-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $50.00-40.00/kg2025-03-07
- CAS:
- 106-99-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20Tons