Mertansine: Origin, Pharmacokinetics and Antitumor Activity
General Description
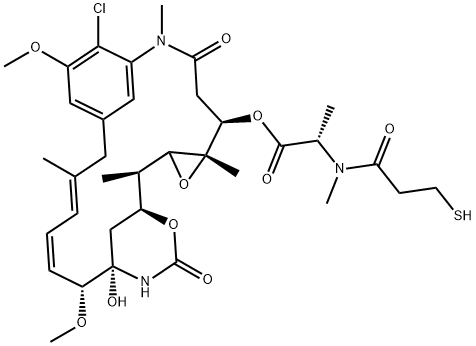
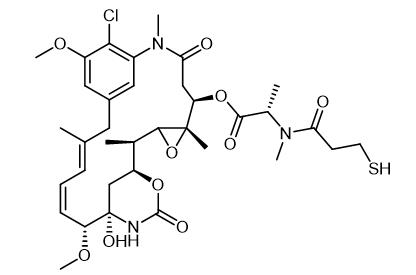
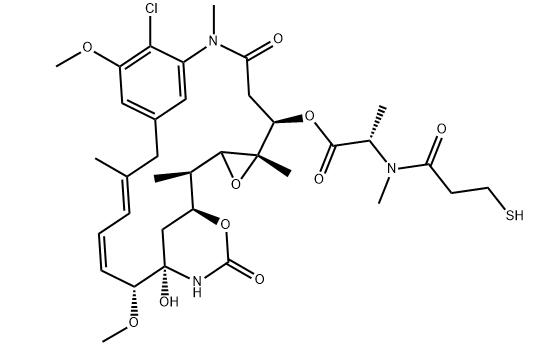
Mertansine, discovered from Maytenus ovatus, is a potent ansa macrolide with significant antileukemic properties. Mertansine disrupts microtubule dynamics, crucial for cell division, thus inhibiting cancer cell proliferation. This discovery highlighted the importance of natural products in drug development, especially for cancer treatment. Mertansine, following intravenous administration, shows complex pharmacokinetics, being rapidly distributed to organs and primarily excreted through feces. Mertansine undergoes extensive metabolism and can influence the metabolism of other drugs by interacting with human liver enzymes. Its potent antitumor activity, due to microtubule disruption, has led to its use in antibody-drug conjugates for targeted cancer therapy, demonstrating the compound's valuable role in advancing cancer treatment strategies.

Figure 1. Mertansine
Origin
In the course of a continuing search for tumor inhibitors from plant sources, researchers found that an alcoholic extract of Maytenus ouatus Loes. showed significant inhibitory activity in vitro against cells derived from human carcinoma of the nasopharynx (KB) and against five standard animal tumor systems. They reported the isolation and structural elucidation of maytansine (l), a novel antileukemic ansa macrolide tumor inhibitors from Maytenus ouatus. Maytansine is the first ansa macrolide shown to contain carbinolamine, epoxide, or aryl halide functions and appears to be the first member of the series reported to show significant in vivo tumor inhibitory activity.1
Pharmacokinetics
DM1, a derivative of maytansine, is the cytotoxic component of the antibody-drug conjugate trastuzumab mertansine (T-DM1). Understanding the disposition and metabolism of DM1 would help to assess (1) any tissue-specific distribution and risk for potential drug-drug interactions and (2) the need for special patient population studies. To this end, the study by Sandhya Girish et. al. determined the disposition and metabolism of DM1 following single intravenous administration of [(3)H]-DM1 in Sprague Dawley rats. Blood, tissues, urine, bile, and feces were collected up to 5 days after dose administration and analyzed for total radioactivity and metabolites. Results showed that radioactivity cleared rapidly from the blood and quickly distributed to the lungs, liver, kidneys, spleen, heart, gastrointestinal tract, adrenal glands, and other tissues without significant accumulation or persistence. The majority of dosed radioactivity was recovered in feces (~100% of the injected dose over 5 days) with biliary elimination being the predominant route (~46% of the injected dose over 3 days). Excretion in urine was minimal (~5% of the injected dose over 5 days). Mass balance was achieved over 5 days.
An analysis of bile samples revealed a small fraction of intact DM1 and a predominance of DM1 metabolites formed through oxidation, hydrolysis, S-methylation, and glutathione and its related conjugates. Collectively, these data demonstrate that DM1 is extensively distributed and quickly cleared from blood, and undergoes extensive metabolism to form multiple metabolites, which are mainly eliminated through the hepatic-biliary route, suggesting that hepatic function (but not renal function) plays an important role in DM1 elimination.2
Antitumor activity
Metastatic triple-negative breast cancer is one of the most devastating cancer types. Systemic chemotherapy is necessary, but its clinical performance is largely limited by severe side effects.
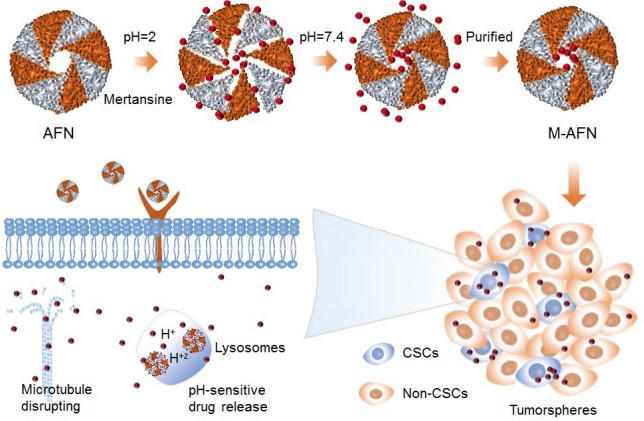
Wei Ran et.al. reported a mertansine prodrug, which could self-assemble into spherical nanoparticles in water and readily convert into active mertansine at the presence of glutathione. The selfassembling mertansine prodrugs (SAMPDs) entered cancer cells via a caveolae-mediated pathway and exhibited potent cytotoxicity. The self-delivering SAMPDs did not cause hemolysis, and more importantly increased maximum tolerated dose (MTD) of mertansine by 8 folds via reducing free mertansine exposure in most of the major organs. SAMPDs improved intratumoral drug exposure and showed dose-dependent antitumor activity. When dosed at MTD, SAMPDs inhibited primary tumor growth and pulmonary metastasis by 80% and 95%, while mertansine dosed at MTD only reduced primary tumor growth and metastasis by less than 50% and 60%, respectively. The results reveal the mechanism of in vivo biotransformation of self-assembling prodrug and highlight the unique advantages of self-assembly prodrug strategy in improving the efficacy and safety of chemotherapy.3
References:
[1] BEN-QUAN SHEN. Non-Clinical Disposition and Metabolism of DM1, a Component of Trastuzumab Emtansine (T-DM1), in Sprague Dawley Rats.[J]. Drug metabolism letters, 2015, 9 2. DOI:10.2174/1872312809666150602151922.[2] WEI RAN . Self-assembling mertansine prodrug improves tolerability and efficacy of chemotherapy against metastatic triple-negative breast cancer[J]. Journal of Controlled Release, 2020, 318: 1-280. DOI:10.1016/j.jconrel.2019.12.027.
You may like
Related articles And Qustion
See also
Lastest Price from Mertansine manufacturers

US $30.00-20.00/BOX2024-03-31
- CAS:
- 139504-50-0
- Min. Order:
- 10BOX
- Purity:
- 99
- Supply Ability:
- 20TONS

US $10.00/KG2021-08-31
- CAS:
- 139504-50-0
- Min. Order:
- 1Kg/Bag
- Purity:
- 99%min
- Supply Ability:
- 20000