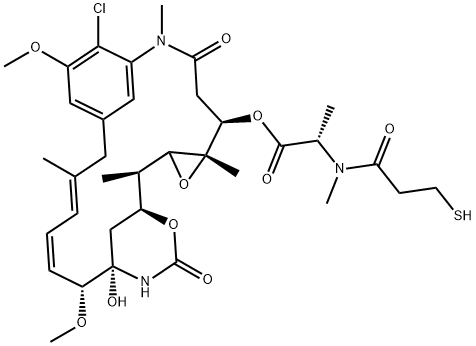
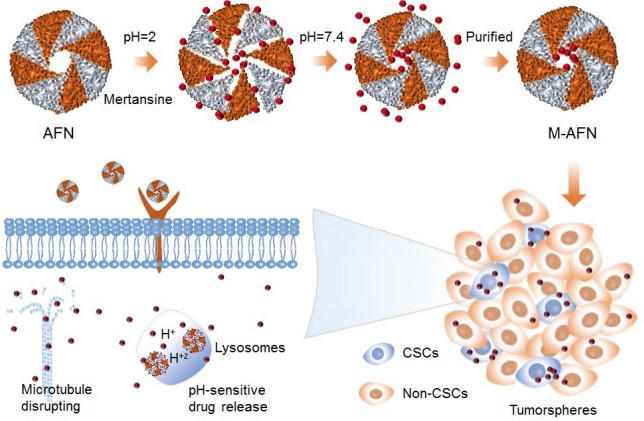
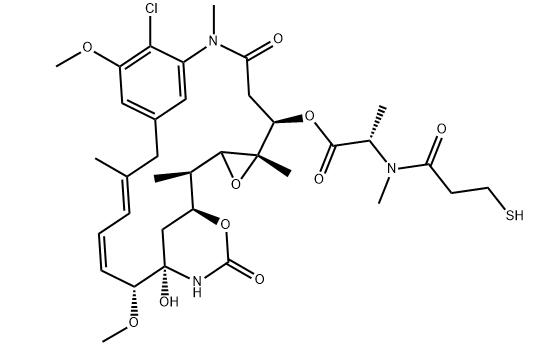
Synthesis and Bioactivity of Maytansine
Maytansine (DM1) is a tubulin inhibitor that binds at the ends of microtubules and inhibits microtubule dynamics. It is an antibody-conjugable maytansinoid used to overcome maytansine-related systemic toxicity and enhance tumor-specific delivery. Maytansine (DM1) is a novel antibody drug, which is composed of trastuzumab and the small molecule microtubule inhibitor DM1, which produces a synergistic anticancer effect. HER2 overexpression is observed in 25%-30% of patients with primary breast cancer, and trastuzumab is a recombinant DNA-derived humanized monoclonal antibody that selectively targets HER-2 extracellular site. It can be used to treat HER-2 positive advanced metastatic breast cancer.
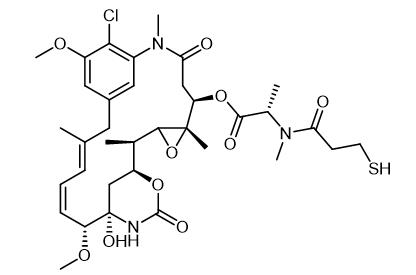
Synthetic routes
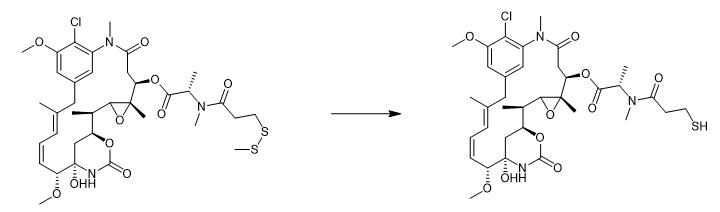
Fig. 2 The synthetic method 1 of Mertansine.
A solution of N 2'-deacetyl-N 2'- (3-methyldithio-1-oxopropyl)- maytansine (2b) (1.95 g, 2.5 mmol) in a mixture of ethyl acetate (140 mL) and methanol (210 mL) was stirred at room temperature under an argon atmosphere and treated with a solution of dithiothreitol (0.95 g, 6.2 mmol) in 0.05 M potassium phosphate buffer (140 mL) at pH 7.5 containing 2 mM ethylenediaminetetraacetic acid (EDTA). The progress of the reaction was monitored by HPLC and was complete in three hours. The reaction mixture was treated with a solution of 0.2 M potassium phosphate buffer (250 mL) at pH 6.0 containing 2 mM EDTA and then extracted with ethyl acetate (3 x 600 mL). The organic layers were combined, washed with brine (100 mL), and then dried over sodium sulfate. Evaporation of the solvent gave a residue of crude thiol-containing maytansinoid 25a. The crude residue was purified by HPLC using a preparative Diazem cyano HPLC column (250 mm x 50 mm, 10 micron particle size) that was equilibrated in a mixture of hexanes/2-propanol/ethyl acetate (78.0:5.5:16.5, v/v/v) and run at a flow rate of 150 mL/min. The desired product 25a eluted as a peak centered at 16 min. The fractions containing the product were evaporated to give 25a as a white solid (76% yield). mp 190-192 °C (dec); 1H NMR (CDCl3) δ 0.84 (3H, s), 1.33 (3H, d, J = 5 Hz), 1.35 (3H, d, J = 5 Hz), 1.60 (3H, s), 1.68 (3H, s), 2.22 (1H, dd, J = 3 and 14 Hz, 2.60-2.82 (2H, m), 2.88 (3H, s), 3.08-3.20 (2H, m), 3.25 (3H, s), 3.39 (3H, s), 3.55 (1H, d, J = 9 Hz), 3.71 (1H, d, J = 12 Hz), 4.02 (3H, s), 4.32 (1H, t, J = 10 Hz), 4.81 (1H, dd, J = 3 and 12 Hz), 5.45 (1H, q, J = 7 Hz), 5.67 (1H, dd J = 9 and 15 Hz), 6.25 (1H, s), 6.47 (1H, dd, J = 11 and 15 Hz), 6.70 (1H, d, J = 1.5 Hz), 6.75 (1H, d, J = 11 Hz), 6.86 (1H, d, J = 1.5 Hz). HRMS calcd for C35H49ClN3O10S (M+ H)+ 738.2827; found, 738.2820; [α]25 D =-113.2, (c = 0.306, CHCl3); extinction coefficient (methanol): ε 280 nm = 5422 M-1 cm-1, ε 252 nm = 25 800 M-1 cm-1 [1].
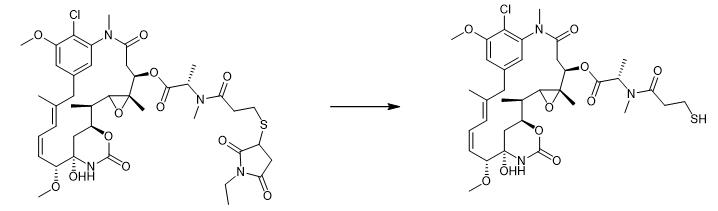
Fig. 3 The synthetic method 2 of Mertansine.
Incubate the synthetic DM1-NEM (8.8 µM in PBS, pH 7.4, 5% DMA)and +/- 10 µM DTT (dithiothreitol) for 18 hours at 37°C. Concentrate the reaction aliquots. Analyze the above mixture by LC-MS. Quantify the maytansinoid products by measuring integrated area under the curve at 252 nm. Compare the above curve to a calibration curve with known standards to obtain the pour product [2].
Determination and Pharmacokinetics
Mertansine, a thiol-containing maytansinoid, is a tubulin inhibitor used as the cytotoxic component of antibody-drug conjugates for the treatment of cancer. Liquid chromatography-tandem mass spectrometry was described for the determination of mertansine in rat plasma. 50-mu L rat plasma sample was pretreated with 25 mu L of 20 mM tris-(2-carboxyethyl)-phosphine, a reducing reagent, and further vortex-mixing with 50 mu L of 50 mM N-ethylmaleimide for 3 min resulted in the alkylation of thiol group in mertansine. Alkylation reaction was stopped by addition of 100 mu L of sildenafil in acetonitrile (200 ng/mL), and following centrifugation, aliquot of the supernatant was analyzed by the selected reaction monitoring mode. The standard curve was linear over the range of 1-1000 ng/mL in rat plasma with the lower limit of quantification level at 1 ng/mL. The intra- and inter-day accuracies and coefficient variations for mertansine at four quality control concentrations were 96.7-113.1% and 2.6-15.0%, respectively. Using this method, the pharmacokinetics of mertansine were evaluated after intravenous administration of mertansine at doses of 0.2, 0.5, and 1 mg/kg to female Sprague Dawley rats [3].
Bioactivity
Inhibits mRNA Expression and Enzyme Activities
Mertansine, a tubulin inhibitor, is used as the cytotoxic component of antibody-drug conjugates (ADCs) for cancer therapy. The effects of mertansine on uridine 5 '-diphospho-glucuronosyltransferase (UGT) activities in human liver microsomes and its effects on the mRNA expression of cytochrome P450s (CYPs) and UGTs in human hepatocytes were evaluated to assess the potential for drug-drug interactions (DDIs). Mertansine potently inhibited UGT1A1-catalyzed SN-38 glucuronidation, UGT1A3-catalyzed chenodeoxycholic acid 24-acyl-beta-glucuronidation, and UGT1A4-catalyzed trifluoperazine N-beta-d-glucuronidation, with K-i values of 13.5 mu M, 4.3 mu M, and 21.2 mu M, respectively, but no inhibition of UGT1A6, UGT1A9, and UGT2B7 enzyme activities was observed in human liver microsomes. A 48 h treatment of mertansine (1.25-2500 nM) in human hepatocytes resulted in the dose-dependent suppression of mRNA levels of CYP1A2, CYP2B6, CYP3A4, CYP2C8, CYP2C9, CYP2C19, UGT1A1, and UGT1A9, with IC50 values of 93.7 +/- 109.1, 36.8 +/- 18.3, 160.6 +/- 167.4, 32.1 +/- 14.9, 578.4 +/- 452.0, 539.5 +/- 233.4, 856.7 +/- 781.9, and 54.1 +/- 29.1 nM, respectively, and decreased the activities of CYP1A2-mediated phenacetin O-deethylase, CYP2B6-mediated bupropion hydroxylase, and CYP3A4-mediated midazolam 1 '-hydroxylase. These in vitro DDI potentials of mertansine with CYP1A2, CYP2B6, CYP2C8/9/19, CYP3A4, UGT1A1, and UGT1A9 substrates suggest that it is necessary to carefully characterize the DDI potentials of ADC candidates with mertansine as a payload in the clinic [4].
A phase I study
Purpose: The purpose is to determine the maximum-tolerated dose, assess the toxicities, characterize the pharmacokinetic behavior, and seek preliminary evidence of biological activity of cantuzumab mertansine when administered as a weekly i.v. infusion without interruption. Experimental Design: Patients with incurable solid tumors that expressed the target antigen for cantuzumab mertansine, CanAg, were treated with doses of cantuzumab mertansine ranging from 40 to 138 mg/m(2).
The maximum-tolerated dose was defined as the highest dose at which no more than 1 of 6 patients experienced dose-limiting toxicity. Plasma concentrations of cantuzumab mertansine and total humanized antibody were determined, and area under the plasma concentration-time curve (to the last measured concentration) was calculated. Results: Thirty-nine patients received a total of 280 weekly doses of cantuzumab mertansine. Acute, transient elevation of the hepatic transaminases and reversible fatigue were identified as the dose-limiting toxicities at the highest dose level.
The maximum-tolerated dose was determined to be 115 mg/m(2)/week. Evidence of clinical activity was noted in 3 patients. Pharmacokinetic analyses revealed that the pharmacokinetic variability was moderate, without evidence of dose dependency. Furthermore, the drug had a long terminal half-life (similar to40 h). Conclusions: This study identified a safe and tolerable dose of the novel immunoconjugate prodrug cantuzumab mertansine. The evidence of antitumor activity suggests that additional clinical development is warranted, with a focus on tumors that express high levels of CanAg and which are known to be sensitive to antimicrotubule agents [5].
References
[1] Widdison W C, Wilhelm S D, Cavanagh E E, et al. Semisynthetic maytansine analogues for the targeted treatment of cancer[J]. Journal of medicinal chemistry, 2006, 49(14): 4392-4408.
[2] Fishkin N, Maloney E K, Chari R V J, et al. A novel pathway for maytansinoid release from thioether linked antibody–drug conjugates (ADCs) under oxidative conditions[J]. Chemical Communications, 2011, 47(38): 10752-10754.
[3] Choi W G, Kim J H, Jang H J, et al. Determination of Mertansine in Rat Plasma Using Liquid Chromatography-Tandem Mass Spectrometry and Pharmacokinetics of Mertansine in Rats[J]. Mass Spectrometry Letters, 2020, 11(3): 59-64.
[4] Choi W G, Park R, Kim D K, et al. Mertansine Inhibits mRNA Expression and Enzyme Activities of Cytochrome P450s and Uridine 5′-Diphospho-Glucuronosyltransferases in Human Hepatocytes and Liver Microsomes[J]. Pharmaceutics, 2020, 12(3): 220.
[5] Helft P R, Schilsky R L, Hoke F J, et al. A phase I study of cantuzumab mertansine administered as a single intravenous infusion once weekly in patients with advanced solid tumors[J]. Clinical cancer research, 2004, 10(13): 4363-4368.
Related articles And Qustion
See also
Lastest Price from Mertansine manufacturers

US $30.00-20.00/BOX2024-03-31
- CAS:
- 139504-50-0
- Min. Order:
- 10BOX
- Purity:
- 99
- Supply Ability:
- 20TONS

US $10.00/KG2021-08-31
- CAS:
- 139504-50-0
- Min. Order:
- 1Kg/Bag
- Purity:
- 99%min
- Supply Ability:
- 20000