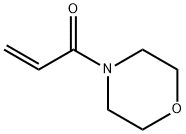
Synthesis, Hazard and Application of N-Acryloylmorpholine
Physicochemical properties
4-Acryloylmorpholine has a melting point of ?35 °C and is liquid at room temperature. At 25 °C, it has a density of 1.122 g/mL. It needs to be stored at 2-8°C.

Fig. 1 The structure of 4-Acryloylmorpholine.
Synthetic routes
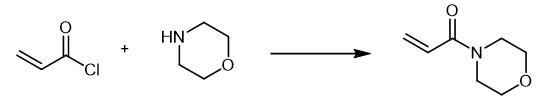
Fig. 2 The synthetic method 1 of 4-Acryloylmorpholine.
Amides XVI-XXI, XXVIII and pentane-1,5-diyl diacrylate (XXXI). General procedure. To a solution of 10.0 mmol of amine (pentane-1,5-diol) and 15.0 mmol of acryloyl chloride in 20 mL of dichloromethane at 0°C under an argon atmosphere was added dropwise while stirring 30.0 mmol of triethylamine in 10 mL of dichloromethane. The reaction mixture was warmed to room temperature and stirred for 6 h. The solvent was removed in a vacuum, the residue was diluted with 20 mL of ether, the precipitated salt Et3N·HCl was filtered off. The mother liquor was evaporated in a vacuum. 4-acryloylmorpholine (XXVIII), Yield: 60-90% [1].
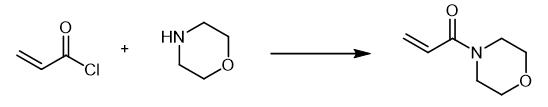
Fig. 3 The synthetic method 2 of 4-Acryloylmorpholine.
A solution of 0.04 mol of the corresponding amine in 20 ml of anhydrous methylene chloride was slowly added at 0-5°C to 0.02 mol of acryloyl chloride in 20 ml of anhydrous methylene chloride. The mixture was stirred for 3 h at room temperature in an inert atmosphere, and the precipitate was filtered off and washed with methylene chloride (2 × 10 ml). The organic layer was washed in succession with 5 ml of water and 5 ml of a saturated solution of NaHCO3 and dried over Na2SO4, the solvent was removed under reduced pressure, and the residue was purified by column chromatography on silica gel using hexane-ethyl acetate (5 : 1 to 1 : 1) as eluent. 4-(Prop-2-enoyl)morpholine (IIe). Yield 1.78 g (63%). IR spectrum, ν, cm-1: 2857, 1647, 1612, 1439, 1263, 1238, 1115, 1038, 953. 1H NMR spectrum, δ, ppm: 3.51-3.73 m (8H, NCH2CH2O), 5.72 d.d (1H, 3-Hcis, 3J = 10.6, 2J = 1.9 Hz), 6.29 d.d (1H, 3-Htrans, 3J = 16.7, 2J = 1.9 Hz), 6.57 d.d (1H, 2-H, J = 16.7, 10.6 Hz). 13C NMR spectrum, δC, ppm: 41.74 and 45.66 (CH2N), 66.22 (CH2O), 126.64 (C2), 127.69 (C3), 164.92 (C1). Mass spectrum, m/z (Irel, %): 141 (36) [M]+, 140 (12), 126 (58), 112 (22), 111 (15), 110 (15), 109 (12), 98 (10), 96 (26), 86 (72), 83 (13), 70 (14), 68 (14), 57 (17), 56 (86), 55 (100), 42 (23) [2].
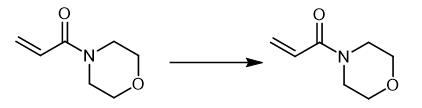
Fig. 4 The synthetic method 3 of 4-Acryloylmorpholine.
Prepare poly(N-acryloylmorpholine) through radical polymerization of N-acryloylmorpholine in the presence of AIBN as initiator and 2-mercaptoethanol as the chain transfer agent. The acidic condition is selected in the system to prevent any hydrogen-transfer addition reaction bySH groups from 2-mercaptoethanol to ACMO double bonds. Dissolve ACMO (4.26 g) and AIBN (1 wt %, 0.0423 g) in a mixed solution of 0.1M acetic acid solution (15 mL) and methyl alcohol (3 mL) in a 100 mL round-bottom flask. Purge the reaction vessel with nitrogen for 30 minutes at 25 °C. Add 2-mercaptoethanol (0.99 mmol, 0.0773 g) to the mixture. Keep the mixture in an oil bath at 60 °C for 24 hours with vigorous stirring. Extract the reaction mixture several times with diethyl ether (4 × 20 mL) to remove excess initiator. Freeze dry the reaction mixture. Purify the crude product by three dissolution/precipitation cycles using dichloromethane as solvent and diethyl ether. 1H NMR (400 MHz, TMS, CDCl3 ppm): δ 1.72 (2nH, m, -CH-CH2-), 2.42-2.68 (1nH, m, -CH-CH2-and 2H, t, -SCH2CH2-), 3.33-3.65 (8nH, t,-CH2-protons in morpholine rings), 4.03 (2H, m,-SCH2CH2OH). IR FT-IR (film; ν (cm-1)): 3485-3560 (-OH stretch), 2857-2967 (-C-H stretch), 1733 (ester-C=O stretch), 1640 (tertiary amide-C=O stretch), 1445 (-CH2-bending), 1230-1275 (C-N stretch) and 1030 -1115 (CO stretch) [3].
Hazard
Gatica-Ortega et al. recently identified an outbreak of occupational allergic contact dermatitis (ACD) involving workers of a Spanish company selling smartphone protective cases from a glue product. A chemical analysis of one glue sample revealed the presence of 4-acryloylmorpholine among other allergens.The same glue is also used to attach tempered glass protective cases to Apple smartwatches. OBJECTIVE: Our objective was to describe a case series of nonoccupational consumer ACD from the previously mentioned Apple smartwatch protective case glue. We evaluated epidemiological and clinical data, as well as patch tests results. Three women were diagnosed with nonoccupational ACD from the adhesive. An annular vesicular inflammatory plaque involving the dorsal aspect of the wrist was initially observed in all. Two of the 3 patients were patch tested with 4-acryloylmorpholine 0.5% with positive strong reactions. Both also strongly reacted to a sample of the glue semiopen tested in a drop of petrolatum. One of them was also positive for various acrylates. 4-Acryloylmorpholine has been identified in an adhesive used to attach protective cases to smartwatches. Nonoccupational ACD have been described to involve consumers of smartwatches. A UV-curable adhesive used to attach protective cases to smartwatches has been considered to be the culprit [4].
Application
Clay-based nanocomposite hydrogel
Although clay-based nanocomposite hydrogels have been widely explored, their instability in hot water and saline solution inhibits their applications in biomedical engineering, and the exploration of clay-based nanocomposite hydrogels in bone defect repair is even less. In this work, we developed a stable clay-based nanocomposite hydrogel using 4-acryloylmorpholine as the monomer. After UV light illumination, the obtained poly(4-acryloylmorpholine) clay-based nanocomposite hydrogel (poly(4-acry)-clay nanocomposite hydrogel) exhibits excellent mechanical properties due to the hydrogen bond interactions between the poly(4-acryloylmorpholine) chains and the physical crosslinking effect of the nanoclay. Besides good biocompatibility, the sustainable release of intrinsic Mg2+ and Si4+ from the poly(4-acry)-clay nanocomposite hydrogel endows the system with excellent ability to promote the osteogenic differentiation of primary rat osteoblasts (ROBs) and can promote new bone formation effectively after implantation. We anticipate that these kinds of clay-based nanocomposite hydrogels with sustained release of bioactive ions will open a new avenue for the development of novel biomaterials for bone regeneration [5].
Copolymers
Luminescent-labeled copolymers of 4-acryloylmorpholine and 2-dialkylaminoethyl methacrylates (2-dimethylaminoethyl methacrylate and 2-diethylaminoethyl methacrylate) of varied compositions and molecular masses were synthesized. Polarized luminescence method was used to study relaxation properties of the polymers in diluted aqueous solutions. The influence of copolymer composition, temperature, pH, ionic strength of solutions on structural and dynamic characteristics of the copolymers in solution was established. The obtained copolymers were used to synthesize silver nanoparticles with an ave
Related articles And Qustion
See also
Lastest Price from 4-Acryloylmorpholine manufacturers

US $2.20-8.80/KG2025-07-08
- CAS:
- 5117-12-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons

US $1.00/kg2025-04-21
- CAS:
- 5117-12-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10mt