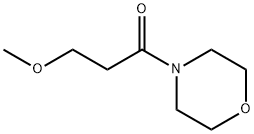
Description
4-Acryloylmorpholine (4-AM) is a water-soluble monomer, an acrylamide derivative containing a heterocyclic tetrahydrooxazine substituent. Homo- and copolymers of 4-АМ have several valuable properties. They are soluble both in water and in many organic solvents such as alcohols, chloroform, tetrahydrofuran, and dioxane, are non-toxic, and are used for solid-phase synthesis of peptides in chromatography, as materials for composite semipermeable membranes, in catalysis, and for biomedical purposes. Various functional groups are introduced into poly-4-AM to expand its application fields. Random copolymers of 4-АМ with carboxylic acids (acrylic, 4-pentenoic, undecenoic, 2-acrylamido-2-methyl-1-propanesulfonic), behaving as anionic polyelectrolytes, and copolymers with N-hydroxysuccinimide ester of acrylic acid, acting as polymeric carriers, have been synthesized[2-3].
Uses
4-Acryloylmorpholine is used in adhesives, UV curable resins, industrial coatings, UV printing ink, oil recovery polymer, medicinal and commodity chemicals.
Synthesis
A
solution of 0.04 mol of the corresponding amine in 20 ml of anhydrous
methylene chloride was slowly added at 0-5??C to 0.02 mol of acryloyl
chloride in 20 ml of anhydrous methylene chloride. The mixture was
stirred for 3 h at room temperature in an inert atmosphere, and the
precipitate was filtered off and washed with methylene chloride (2 ?á 10
ml). The organic layer was washed in succession with 5 ml of water and 5
ml of a saturated solution of NaHCO3 and dried over Na2SO4, the solvent
was removed under reduced pressure, and the residue was purified by
column chromatography on silica gel using hexane-ethyl acetate (5 : 1 to
1 : 1) as eluent. 4-Acryloylmorpholine, Yield 1.78 g (63%).
IR spectrum, |í, cm-1: 2857, 1647, 1612, 1439, 1263, 1238, 1115, 1038,
953. 1H NMR spectrum, |?, ppm: 3.51-3.73 m (8H, NCH2CH2O),
5.72 d.d (1H, 3-Hcis, 3J = 10.6, 2J = 1.9 Hz), 6.29 d.d (1H, 3-Htrans,
3J = 16.7, 2J = 1.9 Hz), 6.57 d.d (1H, 2-H, J = 16.7, 10.6 Hz). 13C NMR
spectrum, |?C, ppm: 41.74 and 45.66 (CH2N), 66.22 (CH2O), 126.64 (C2),
127.69 (C3), 164.92 (C1). Mass spectrum, m/z (Irel, %): 141 (36) [M]+,
140 (12), 126 (58), 112 (22), 111 (15), 110 (15), 109 (12), 98 (10), 96
(26), 86 (72), 83 (13), 70 (14), 68 (14), 57 (17), 56 (86), 55 (100),
42 (23).
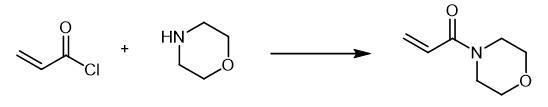
Fig. The synthetic method 2 of 4-Acryloylmorpholine
References
[1] PRIETO O, LAM H W. Cobalt-catalyzed reductive Mannich reactions of 4-acryloylmorpholine with N-tosyl aldimines??[J]. Organic & Biomolecular Chemistry, 2007. DOI:10.1039/B715839D.
[2] T. Nekrasova. “pH- and thermosensitive copolymers of 4-acryloylmorpholine and 2-dialkylaminoethyl methacrylates and silver-containing nanocomposites based on these copolymers.” Materials Today Communications (2019).
[3] Yu. I. Zolotova. “Copolymers of 4-Acryloylmorpholine with 2-Dimethyl- and 2-Diethylaminoethyl Methacrylate and Silver-Containing Nanocomposites Based on Them.” Russian Journal of Applied Chemistry 91 4 (2018): 623–628.




 Fig. The synthetic method 2 of 4-Acryloylmorpholine
Fig. The synthetic method 2 of 4-Acryloylmorpholine