Meloxicam: applications, preparation method and pharmacology
Introduction
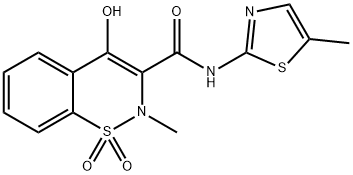
Meloxicam is also konwn as 4-hydroxy-2-methyl-N-(5-methyl-2-thiazolyl)-2H-1,2-benzothiazine3-carboxamide 1,1-dioxide. Meloxicam is a pale yellow, crystalline, powder. (Figure 1) It is practically insoluble in water, soluble in dimethylformamide, and very slightly soluble in 96%aqueous ethanol.The compound exhibits polymorphic dependent solubility as well. [1]

Mechanism of action
Meloxicam inhibits prostaglandin synthetase (cylooxygenase 1 and 2) enzymes leading to a decreased synthesis of prostaglandins, which normally mediate painful inflammatory symptoms. As prostaglandins sensitize neuronal pain receptors, inhibition of their synthesis leads to analgesic and inflammatory effects. Meloxicam preferentially inhibits COX-2, but also exerts some activity against COX-1, causing gastrointestinal irritation.[2]
Uses and applications
Meloxicam, an oxicam derivative, is a nonsteroidal anti-inflammatory drug (NSAID) that is reported to be a selective inhibitor of cyclooxygenase-2 (COX-2). Meloxicam is used in the management of rheumatoid arthritis, for the short-term symptomatic treatment of acute exacerbations of osteoarthritis, and for the symptomatic treatment of ankylosing spondylitis. It may also be used in the treatment of juvenile idiopathic arthritis. In the treatment of rheumatoid arthritis and ankylosing spondylitis, meloxicam is given in a usual oral dose of 15 mg daily as a single dose. Those with an increased risk of adverse reactions should be started on 7.5 mg daily. A dose of 7.5 mg daily is recommended for long-term treatment in the elderly. In the treatment of acute exacerbations of osteoarthritis the usual oral daily dose of meloxicam is 7.5 mg, increased if necessary to a maximum of 15 mg daily given as a single dose. Meloxicam may be given by rectal suppository in doses similar to those used orally but use should be limited to the shortest time possible.[3]
Meloxicam, in combination with bupivacaine, is indicated for postsurgical analgesia in adult patients for up to 72 hours following soft tissue surgical procedures, foot and ankle procedures, and other orthopedic procedures in which direct exposure to articular cartilage is avoided.
Methods of preparation
E. Vigano et al. [4] invented a procedure for preparation and purification of meloxicam. The crude meloxicam used in the present invention is obtained by making the 4-hydroxy-2-methyl-2H-1,2-benzothiazine-3-carboxylate of ethyl-1,1-dioxide react with 2-amino-5-methyl-thiazole in Xylene according to Scheme 1 below:The reaction is carried out under reflux in the presence of molecular sieves 4A or 5A suited to adsorb the ethanol which is formed. The crude meloxicam is isolated by filtration and used wet in the purification process in the present invention. The Applicant has also found that with the process of the present invention it is possible to lower the impurity composed of ethyl amide to values lower than 0.05% starting from crude meloxicam coming from the above-mentioned reaction containing the above mentioned impurity up to values of about 1%. A further advantage of the process object of the present invention lies in the use of non-toxic solvents,thus avoiding the use of toxic solvents such as chlorinated Solvents and dimethylformamide, which are the customary crystallization solvents for meloxicam.

Pharmacology
F. Engelhardt [5] reviewed the key pharmacological findings determined for meloxicam. Unlike the other established NSAID compounds,meloxicam preferentially inhibits inducible COX-2 in guinea-pig peritoneal macrophages and human COX-2 in COS cells. Compared with other NSAIDs, meloxicam is the most potent inhibitor of prostaglandin biosynthesis in pleural and peritoneal exudate, but only a weak inhibitor in the gastric tract and kidney. Ulcerogenicity in the rat stomach is weak in relation to anti-inflammatory potency, resulting in a high therapeutic index. The high anti-inflammatory potency of Meloxicam combined with its good tolerability can be explained by its preferential inhibition of COX-2. In adjuvant arthritis rats, meloxicam inhibits not only paw swelling, but also bone and cartilage destruction and systemic signs of disease. It inhibits leukocyte migration, but has no effect on leucotriene B4 or C4. Meloxicam shows a long-lasting anti-inflammatory and analgesic effect on inflammatory pain and reduces pyrogen-induced fever, but has no central nervous system effects.
In another study, G. Engelhardt and coworkers [6] investigated the anti-inflammatory, analgesic and antipyretic properties of meloxicam in avariety of animal models, comparing its properties with the properties of piroxicam, diclofenac, indomethacin and several other NSAIDs. It was concluded that meloxicam had no effect in a model of visceral distention pain. In common with other NSAIDs, meloxicam had no influence on the body temperature of normothermic rats in the anti-inflammatory dose range, but did reduce yeast-induced fever in the rat in a dose-dependent manner. Like piroxicam, meloxicam had a uricosuric effect on rats treated with oxonic acid. Low-dose meloxicam inhibited both bradykinin-induced and PAF-induced bronchospasm in the guinea-pig, but had no effect on acetylcholine-induced bronchospasm. Piroxicam had greater ulcerogenic effects in the rat stomach than meloxicam. The therapeutic range of meloxicam in the rat, with regard to inhibition of adjuvant arthritis, was several times greater than that of piroxicam, indomethacin, diclofenac, andnaproxen.[3]
References
[1] British Pharmacopoeia. vol. II, Her Majesty’s Stationary Office, London, United Kingdom, 2016, p. 2373.
[2] A. Bekker, C. Kloepping, S. Collingwood. Meloxicam in the management of post-operative pain: Narrative review. J Anaesthesiol Clin Pharmacol. 2018,34(4):450-457.
[3] N. Y. Khalil, , & K. F. Aldosari. Meloxicam. Profiles of drug substances, excipients, and related methodology, 2020, 45: 159–197.
[4] E. Vigano, L. D’erba, E. Landonio, Rescaldina, United States Patent Application No.:US 2006/0116514 A1, Jun. 1, 2006.
[5] G. Engelhardt, Pharmacology of meloxicam, a new non-steroidal anti-inflammatorydrug with an improved safety profile through preferential inhibition of COX-2, Br.J. Rheumatol. 1996,35 (1): 4-12.
[6] G. Engelhardt, D. Homma, K. Schlegel, R. Utzmann, C. Schnitzler, Anti-inflammatory, analgesic, antipyretic and related properties of meloxicam, a new non-steroidalanti-inflammatory agent with favorable gastrointestinal tolerance, Inflamm. Res. 1995,44(10): 423-433.
You may like
Related articles And Qustion
Lastest Price from Meloxicam manufacturers

US $5.00-0.50/KG2025-06-13
- CAS:
- 71125-38-7
- Min. Order:
- 0.10000000149011612KG
- Purity:
- 99% hplc
- Supply Ability:
- 5000kg

US $5.00-0.50/KG2025-05-08
- CAS:
- 71125-38-7
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS