Is meloxicam safe to use?
Introduction
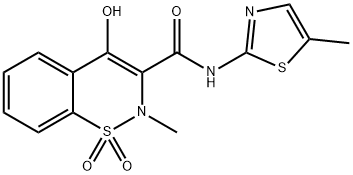
Meloxicam is an oxicam derivative and a member of the enolic group of NSAIDs. Although it possesses some COX-2 selectivity (e.g., preferential inhibition of COX-2 over COX-1 is most pronounced at lower doses), meloxicam is not considered a selective COX-2 inhibitor by the US FDA. Meloxicam is available in 7.5-mg tablets. The recommended starting and maintenance dose for osteoarthritis is 7.5 mg orally once daily with a maximum daily dose of 15 mg. Because it is associated with fewer GI side effects, unlike most NSAIDs, meloxicam may be given without regard to meals. It is available as an injectable preparation, an oral suspension and a chewable tablet.
Clinical applications
Meloxicam is indicated for managing chronic soft tissue or musculoskeletal pain, including osteoarthritis in dogs. It is also effective for managing perioperative pain in dogs undergoing orthopedic or soft tissue surgery.
Meloxicam was recently registered in some markets for use in cats for short-term (1 d) treatment. It appears to have similar efficacy to ketoprofen.
Meloxicam is approved in Europe for use in horses for the alleviation of inflammation and relief of pain in both acute and chronic musculoskeletal disorders.
Safety
In a pharmacokinetic study in rats, Busch et al. (1998) found that meloxicam is well absorbed following oral dosing and that the plasma concentration-time profiles were comparable in rats, dogs and humans. They also noted that elimination of the drug was considerably slower in female than male rats and in albino versus hooded rats. The primary means of elimination was renal excretion. They also found that meloxicam appears in the milk and passes the placenta, with levels in the liver of day 18 fetuses being about one-third to one-fifth in the maternal liver. Villegas et al. (2002) compared meloxicam with piroxicam at two doses and found equivalent levels of gastric ulceration.
Meloxicam, given at 7.5 mg/kg PO for 14 days, resulted in the death of 43% of the rats, with weight loss and diarrhea as the primary clinical signs. Using a lower dose of 3.75 mg/kg PO for 28 days, no deaths were reported and hematological and liver enzymes, blood urea nitrogen (BUN) and creatinine were within normal limits except a mild elevation of aspartate aminotransferase (AST). The presence of gastric lesions within 9 hours following a single oral dose of meloxicam at 7.5 and 15 mg/kg was again confirmed by Villegas et al. (2004). It should be noted that the doses in these studies are far higher than those recommended for osteoarthritis in dogs and at least 50% higher than the dose used by Roughan et al. (2004); thus, their clinical relevance in terms of adverse effects in rats is questionable.
For short-term use, it's perfectly safe to take meloxicam daily, as long as you aren't allergic and it's not contraindicated with any medical conditions. Meloxicam is typically used for 10 to 14 days to manage pain, but treatment duration can vary depending on the specific medical condition being treated. Your healthcare provider will likely take you off it once your discomfort is under control.
However, meloxicam is not typically considered safe for daily long-term use. This means that taking it every day indefinitely is not recommended. However, if a healthcare professional has prescribed meloxicam for a long period, they likely weighed the risks versus benefits before going this route and will be checking in on you regularly. Your provider may lengthen or shorten the duration of your treatment with meloxicam based on how much pain you are in, how well-controlled your condition is, and other health conditions you may have, including pre-existing heart or stomach issues.
Related articles And Qustion
See also
Lastest Price from Meloxicam manufacturers

US $5.00-0.50/KG2025-06-13
- CAS:
- 71125-38-7
- Min. Order:
- 0.10000000149011612KG
- Purity:
- 99% hplc
- Supply Ability:
- 5000kg

US $5.00-0.50/KG2025-05-08
- CAS:
- 71125-38-7
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS