Loxoprofen:pharmacodynamic properties,pharmacokinetic properties and potential drug interactions
Introduction
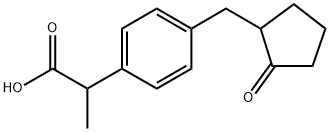
Loxoprofen is chemically and structurally a propionic acid derivative and is a non-steroidal anti-infammatory drug (NSAID) in terms of pharmacology. Loxoprofen is a prodrug type NSAID that has the characteristic of exhibiting higher pharmacological activity than the parent form when reduced and converted to alcoholic form in the body. In the chemical structure of loxoprofen, cyclopentane has a carbonyl group, which is reduced by carbonyl reductases in the body (especially in the liver) to produce alcoholic metabolites. And the carbon at the alcohol formation site is a chiral center and can be classified into ‘trans’ and‘cis’depending on the hydroxyl group configuration of the loxoprofen alcoholic metabolite. According to reports, the degree of anti-inflammatory and analgesic effects by loxoprofen exposure is mainly due to trans-alcohol loxoprofen. The pharmacological activities of cis-alcohol loxoprofen and parent loxoprofen are reported to be almost negligible, less than 1/10 of that of trans-alcohol loxoprofen. The trans-alcohol loxoprofen non-selectively inhibits cyclooxygenase (COX) enzymes involved in the production of mediators (such as prostaglandins, prostacyclins, and thromboxane) that cause fever, inflammation, and pain in the body. Therefore, not only COX-2 inhibition induced by human cells post-injury or due to other stimuli, but also COX-1, which has cellular housekeeping functions such as mucus production and platelet aggregation, can be inhibited by loxoprofen exposure. As a result, sustained high levels of loxoprofen exposure, like other NSAIDs, can cause serious side effects such as erosive gastritis and bleeding.Loxoprofen is clinically used for a variety of indications,including systemic inflammatory diseases after surgery or trauma, as well as chronic rheumatoid arthritis, osteoarthritis, back pain, and acute upper respiratory tract inflammation. The general clinical use of loxoprofen in healthy adults suffering from temporary inflammation and pain is 60 mg tablets administered twice or three times a day, and it has been reported that the total daily dose should not exceed 180 mg. And loxoprofen usually requires continuous administration depending on the patient's prognosis, for an average of 7 days to a year, until the symptoms of inflammation and pain are alleviated.[1] Figure 1 lists the structure and characteristics of Loxoprofen.[2]
Pharmacodynamic Properties of Loxoprofen
Loxoprofen is a non-selective inhibitor of cyclooxygenase enzymes, which are responsible for the formation of various biologically active pain, fever, and inflammatory mediators. These include prostaglandins, prostacyclin, thromboxane, and arachidonic acid. After oral or topical administration, the prodrug-type NSAID loxoprofen undergoes conversion to its active trans-alcohol(trans-OH) metabolite, which potently suppresses prostaglandin biosynthesis via nonselective inhibition of COX enzymes. In vitro, this trans-OH metabolite showed dose-dependent inhibition of human COX-1 and COX-2 enzymes, while loxoprofen did not appear to inhibit either COX enzyme.In animal studies, oral and topical (hydrogel patches) loxoprofen were shown to have anti-inflammatory and analgesic effects that were similar to or greater than those of other commercially available NSAIDs, including indometacin, ketoprofen, flurbiprofen and felbinac. For instance, in rats with carrageenan-induced paw oedema, the anti-inflammatory effect of loxoprofen hydrogel patches was significantly (p<0.01) greater than that of indometacin or felbinac patches, and not significantly different from that of ketoprofen or flurbiprofen patches. In addition, the analgesic effect of loxoprofen hydrogel patches in rats with yeast-induced paw inflammation was significantly (p<0.01) greater than that of indometacin, ketoprofen or felbinac patches,and not significantly different from that of flurbiprofen patches.
In patients with type 2 diabetes and overt nephropathy,short-term loxoprofen topical therapy for knee and/or low back pain did not significantly affect renal function or blood pressure. In these patients, loxoprofen 100 mg tape once daily for five days significantly (p<0.01)reduced visual analogue scale (VAS) pain scores without significantly affecting urinary prostaglandin E2 levels or estimated glomerular filtration rate (eGFR).[3]
Pharmacokinetic Properties of Loxoprofen
Loxoprofen was rapidly absorbed after a single oral dose of loxoprofen 60 mg in healthy adult volunteers, with peak plasma concentrations of loxoprofen and its active trans-OH metabolite being reached after ≈30 and ≈50 min. After a single dermal application of four 1 % loxoprofen patches (400 mg) in healthy volunteers,peak plasma concentrations of loxoprofen and its metabolites were reached at 4 and 6 h. In healthy volunteers, ≈10 % of a single topical dose of 1% loxoprofen (100 mg) was estimated to have been transferred into the body over 12h. During multiple-dose administration of two 1% loxoprofen patches (200 mg)once daily for 5 days, steady-state plasma concentrations of loxoprofen and its metabolites were reached on day 4–5.[3]
Mechanism of action
Loxoprofen itself is a prodrug and carries little-to-no pharmacological activity - it is rapidly metabolized to its trans-alcohol form, which is a potent and non-selective inhibitor of cyclooxygenase. Cyclooxygenase (COX) is present in 2 forms, COX-1 and COX-2, with each serving different functions. COX-1 is present in human cells and is constitutively released, performing cellular housekeeping functions such as mucus production and platelet aggregation. COX-2 is induced in human cells post-injury or due to other stimuli, is triggered to appear in large quantities at the sites of injury/stimuli, and is ultimately responsible for the mediation of inflammation and pain.
Loxoprofen's active metabolite inhibits both COX isoforms, resulting in reduced expression of several mediators of pain, inflammation, and fever (e.g. prostaglandins, prostacyclin, thromboxane, etc).
Potential Drug Interactions
Loxoprofen is a water-soluble drug that is not metabolizedby cytochrome P450 (CYP) enzymes. In vitro, loxoprofen did not affect the metabolism of drugs that are substrates of CYP1A1/2, CYP2A6, CYP2B6, CYP2C8/9,CYP2C19, CYP2D6, CYP2E1 or CYP3A4 enzymes. Nevertheless, similar to other NSAIDs, drug interactions may occur during concomitant use of oral loxoprofen and vitamin K antagonists (e.g. warfarin), sulfonylureas (e.g.tolbutamide), fluoroquinolones (e.g. enoxacin), methotrexate, lithium, thiazide diuretics or antihypertensive agents, although no clinically relevant drug interactions have been reported during topical loxoprofen therapy.Consult local prescribing information for further information regarding drug interactions with loxoprofen.[3]
References
[1] Jang JH, Kang HS, Jeong SH. Population Pharmacokinetics of Loxoprofen and its alcoholic metabolites in healthy Korean men. Daru. 2024;32(2):631-648. doi:10.1007/s40199-024-00533-y
[2]Alqarni M, Namazi NI, Alshehri S, et al. Solubility Optimization of Loxoprofen as a Nonsteroidal Anti-Inflammatory Drug: Statistical Modeling and Optimization. Molecules. 2022;27(14):4357. Published 2022 Jul 7. doi:10.3390/molecules27144357
[3]Greig SL, Garnock-Jones KP. Loxoprofen: A Review in Pain and Inflammation. Clin Drug Investig. 2016;36(9):771-781. doi:10.1007/s40261-016-0440-9
You may like
Lastest Price from Loxoprofen manufacturers

US $5.00-0.50/KG2025-05-08
- CAS:
- 68767-14-6
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS

US $0.00/KG2025-04-21
- CAS:
- 68767-14-6
- Min. Order:
- 1KG
- Purity:
- 99%min
- Supply Ability:
- 500kg/month