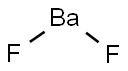
Lewis structure of Barium fluoride
The Lewis structure of barium fluoride (BaF2) is similar to CaF2. It is composed of a central barium atom (Ba) and two non-central fluorine atoms (F). BaF2 is an ionic compound with an ionic bonding type. The Lewis structure of BaF2 is shown below:
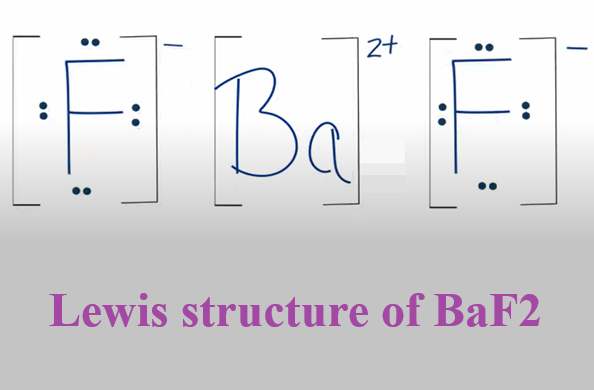
When drawing the Lewis structure diagram of BaF2, the valence electrons of the atoms must be determined first. The valence electrons of barium and fluorine are 2 and 7 respectively. The electronegativity of fluorine atom is less than that of barium atom, so barium atom is the central atom and fluorine atom is the non-central atom. In order to achieve a stable octet, the barium atom loses 2 valence electrons, while the fluorine atom only needs one more electron to form an octet, so the two fluorine atoms each accept one electron to form a stable structure. That is, the two core electrons (outermost electrons) of barium are transferred to the fluorine atom to form a stable BaF2. In this process, the Ba atom becomes a Ba ion (Ba2+) and the fluorine atom becomes a fluorine ion (F-). The oppositely charged ions combine to form the ionic compound barium fluoride (BaF2).
You may like
See also
Lastest Price from Barium fluoride manufacturers

US $10.00/KG2025-04-21
- CAS:
- 7787-32-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00-0.00/BAG2024-11-19
- CAS:
- 7787-32-8
- Min. Order:
- 1BAG
- Purity:
- ≥99%
- Supply Ability:
- 1000 Tons