What is Boron trichloride used for?
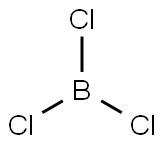
Boron trichloride (chemical formula: BCl3) is an inorganic compound commonly used in dry etching and chemical vapor deposition (CVD) processes in semiconductor manufacturing. It is a colorless gas at room temperature with a strong pungent odor and is sensitive to humid air because it hydrolyzes to produce hydrochloric acid and boric acid.
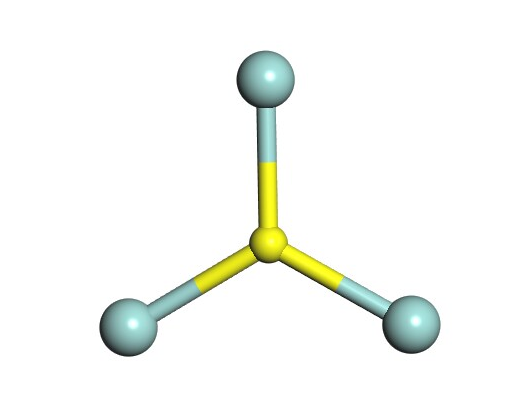
Molecular structure of BCl3
In boron trichloride, B is +3 and Cl is -1. The boron atom covalently bonds with each chlorine atom to form three B-Cl covalent bonds. The electron cloud in the molecule is evenly distributed between the boron and chlorine atoms, making the molecule a planar triangle. The boron atom is located at the center of the molecule, surrounded by three chlorine atoms evenly distributed, and the angle between each two B-Cl bonds is 120°.
What is BCl3 used for?
In the semiconductor industry, boron trichloride is mainly used for dry etching of aluminum and as a dopant to form P-type regions on silicon wafers. It can also be used to etch materials such as GaAs, Si, AlN, and is used as a boron source in some specific applications. In addition, boron trichloride is also widely used in metal processing, glass industry, chemical analysis, and laboratory research.
Safety of Boron Trichloride
Boron trichloride is corrosive and toxic, causing serious damage to the eyes and skin. It hydrolyzes in moist air and releases toxic hydrogen chloride gas. Therefore, proper safety measures need to be taken when handling boron trichloride, including wearing protective clothing, goggles and respiratory protection equipment, and operating in a well-ventilated environment.