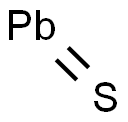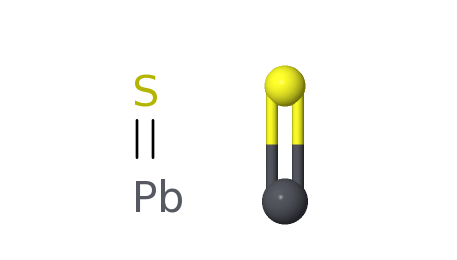Is PbS Soluble in water?
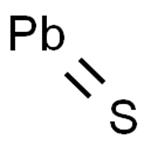
Lead(II) sulfide is an inorganic compound with the formula PbS. PbS, also known as galena, is lead's principal ore and most important compound. It is a semiconducting material with niche uses.

It is a soft, silvery to black, irregularly shaped crystalline powder that is stable at ambient temperature. Lead(II) sulfide is an inert compound that is a semiconductor, photoconductor, and infrared radiation material. These properties lend to its use in electronic components. It is used in semiconductors, ceramic glaze, photoconductive cells, infrared detectors, transistors, humidity sensors, catalysts, mirror coatings, high-temperature lubricants, and blue pigments.
It is a metal sulfide that, like other sulfides in its neighboring spots in the periodic table, is also only sparingly soluble in water. At room temperature, only a small amount of PbS will dissolve in water, resulting in a very low concentration of dissolved Pb2+ and S2- ions. This solubility is affected by factors such as temperature, pH, and the presence of other substances in the water. It forms a black precipitate in the water. PbS is not soluble in water, but it dissolves in nitric acid and dilutes hot HCl. Any "dissolution" process in acids (hydrochloric acid, nitric acid, aqua regia) is accompanied by a chemical reaction whereby H2S is liberated, and the corresponding soluble Pb2+ salts are formed. In nature (soil, minerals), PbS is most likely formed under hydrothermal conditions.
However, it's important to note that lead compounds, including PbS, are highly toxic. Therefore, handling and disposing of lead compounds with proper precautions and in accordance with applicable regulations is advisable.
You may like
Related articles And Qustion
See also
Lastest Price from Lead(II) sulfide manufacturers
US $0.00-0.00/KG2025-04-04
- CAS:
- 1314-87-0
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 1ton
US $0.00-0.00/KG2024-09-04
- CAS:
- 1314-87-0
- Min. Order:
- 1KG
- Purity:
- 99.0%
- Supply Ability:
- 10000KG