Chloroprene: Properties, Production process and Uses
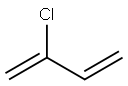
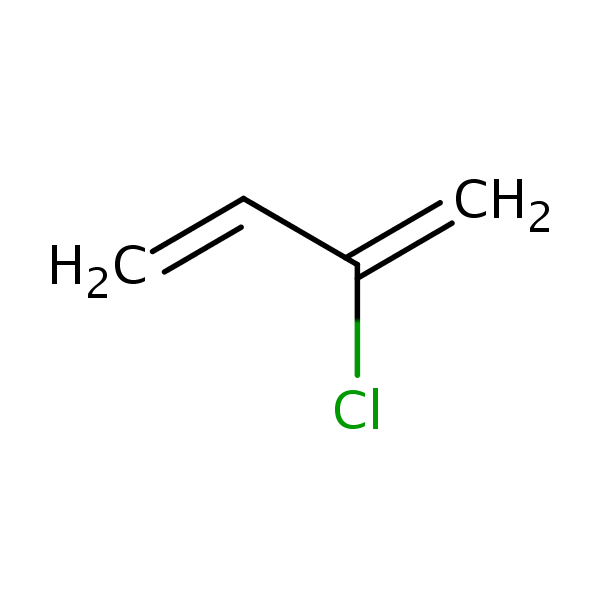
Chloroprene, also known as 2-chloro-1,3-butadiene, 2-chlorobutadiene, 2-chlorobuta- 1,3-diene, β-chloroprene, chlorobutadiene, 2-chloor-1,3-butadieen, 2-chlor-1,3- butadien, β-chlorobutadiene and 2-chloroprene, is a derivative of vinylacetylene. Molecular formula: C4H5Cl, molecular weight: 88.535. The chemical structure is:
Properties of Chloroprene
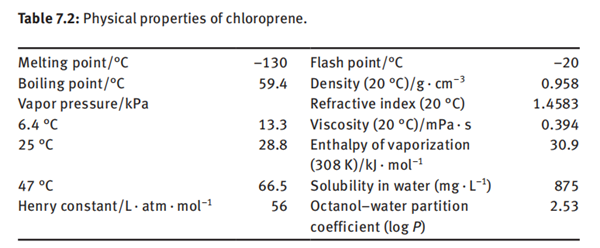
Chloroprene is a colorless and volatile synthetic liquid that has a pungent ether-like odor. It is soluble in ethanol and diethyl ether, miscible with acetone and benzene, and slightly soluble in water. Other physical properties of chloroprene are given in Table 7.2.
Process for manufacture of chloroprene
At present, there are two industrial processes for production of chloroprene in the
world: acetylene route and butadiene route. In China, all the chloroprene is produced
by acetylene route, yet butadiene route dominates the production of chloroprene in
other countries.
The production of chloroprene by acetylene route includes two basic processes: synthesis of vinylacetylene from acetylene and synthesis of chloroprene from vinylacetylene. In the presence of catalyst solution composed of cuprous chloride and an alkali metal salt, acetylene undergoes dimerization reaction to produce vinylacetylene and various by-products such as divinylacetylene, acetaldehyde and vinyl chloride. Pure vinylacetylene is obtained by absorption, desorption and fractional distillation.
Vinylacetylene undergoes an addition reaction with hydrogen chloride in the presence of cuprous chloride catalyst in hydrochloric acid solution to yield chloroprene:
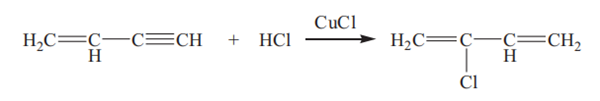
Some by-products such as methyl vinyl ketone and 1-chloro-1,3-butadiene are also generated. The unreacted vinylacetylene is recovered by fractional distillation and recycled. The crude product is cooled and refined to obtain the polymerization-grade chloroprene.
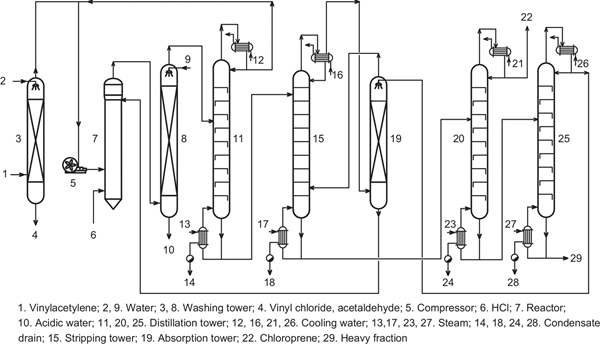
The process flow diagram for production of chloroprene is shown in Figure 7.3. Vinylacetylene was washed by water in the washing tower and introduced into the reactor filled with the catalyst solution by a compressor, and hydrogen chloride is simultaneously introduced into the reactor. The reaction was carried out at a temperature of 40 to 45°C. The reaction mixture was discharged from the top of the reactor and introduced into the fractional distillation tower. The top product of the tower was the unreacted vinylacetylene, which returned to the reactor. The bottom product of the tower was the crude chloroprene, which was subjected to stripping, absorption and fractional distillation to obtain the finished product of chloroprene.
Uses of chloroprene
Chloroprene is mainly used for production of neoprene by free radical emulsion polymerization. The polymerization of chloroprene is initiated using potassium persulfate. Zinc oxide and thioureas are used for crosslinking of individual polymer strands.