Toxicity of Chloroprene
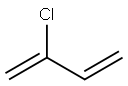
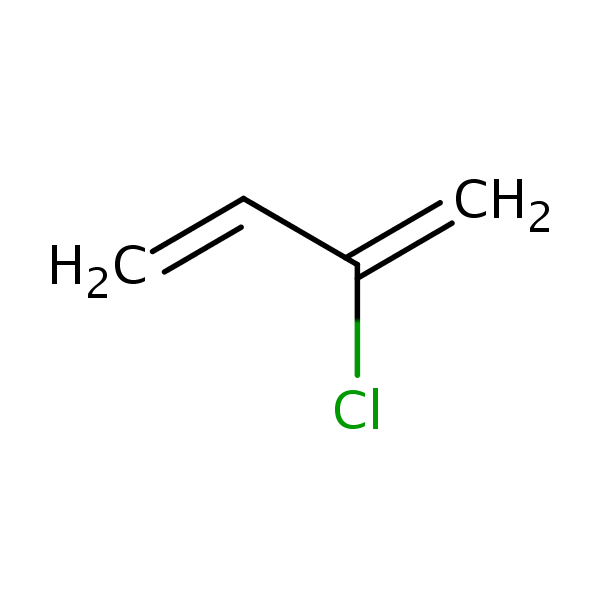
Chloroprene, 2-chloro-1,3-butadiene, is a colorless, volatile
synthetic liquid that has a pungent ether-like odor. Synthesis
of chloroprene was first reported by chemists of the E. I. du
Pont de Nemours Company in 1931 following studies of
acetylene polymerization with the objective of producing
synthetic rubber. The chloroprene monomer differs from
isoprene, the fundamental monomer of natural rubber, only
by substitution of chlorine for the methyl group of isoprene.
Chloroprene was observed to polymerize much more
quickly than did isoprene. In industrial processes prior to
1960, chloroprene was produced in relatively high yields by
reacting vinyl acetylene with hydrogen chloride. Today,
chloroprene is produced more efficiently by chlorination of
1,3-butadiene.

Characteristic
When compared with natural rubber the chloroprene synthetic polymer, polychloroprene, was noted to be much denser, more resistant to water and hydrocarbon solvents, less permeable to many gases, and was more resistant to degradation by oxygen, ozone, hydrogen chloride, hydrogen fluoride, and other chemicals. Due to desirable physical and chemical properties, polychloroprene and its latex polymers are produced in quantities exceeding 200 000 metric tons at a limited number of facilities around the world. Chloroprene production is closely tied to demand for polychloroprene.
Uses
More than 90% of chloroprene produced annually is used to make polychloroprene, which constitutes the widely known DuPont product Neoprene. This solvent-resistant elastomer, made by free radical initiated polymerization of chloroprene, is used to make many automotive rubber products, such as tires, hoses, and belts. Polychloroprene is formulated into adhesives and latex emulsions for dip coated goods. Other products made from polychloroprene are rubber personal protection garments including gloves, shoes, and wetsuits. Polychloroprene is used in conveyor and transmission belts, sealing materials, and electrical insulating materials.
Environmental Fate
Chloroprene is not known to occur naturally. It is not widely distributed in the environment due its reactivity and its use at a limited number of facilitiesworldwide. Industrial productionof chloroprene is accomplished in sealed reactor systems with very limited fugitiveemissions.Polymerizationprocesses are designed to be sealed, but must be opened to remove and manipulate formed polymer. Such opening causes most environmental release of chloroprene, the majority of which enters the atmosphere. From National Library of Medicine Toxics Release Inventory 2010 data, more than 270 000 pounds of chloroprene was released into the environment. Of that amount more than 97% was released into air at one site in Louisiana, USA.
Mechanism of toxicity
The mechanisms of chloroprene toxicity differ depending on the extent and duration of exposure. An acute exposure to large concentration causes central nervous system depression typical of exposure to hydrocarbons. Organ and cellular toxicities due to reactions of chloroprene or metabolites with cellular constituents are evident. Detailed mechanisms of acute toxicities such as formation of edema and hemorrhage in tissues following large exposures are not yet known. Chronic toxicity is related to the mutagenic potential of oxidative metabolites of chloroprene, as these react with specific nucleoside sites in DNA.