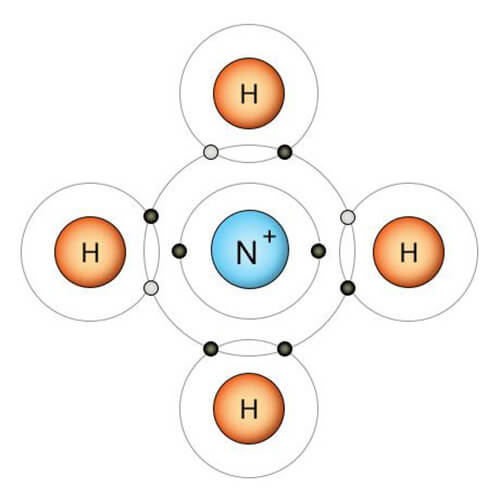
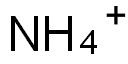Is NH4+ a strong acid?
Mar 20,2024
NO, NH4+ (ammonium ion) is not a strong acid. NH4+ is the conjugate acid of the weak base ammonia (NH3). It is a weak acid. This is because they are in equilibrium in solution. It partially supplies protons (H+) in aqueous solution, resulting in a low concentration of H+ ions and a higher pH than strong acids. This means that there is always some NH3 and NH4+ present in the equilibrium between NH3 and NH4+.