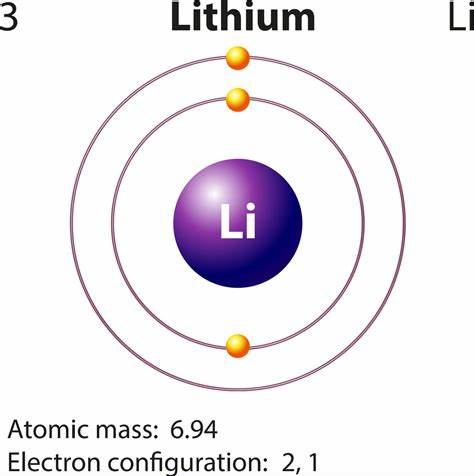
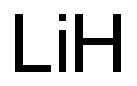What is the oxidation state of Li?
Mar 20,2024
The oxidation state of Lithium (Li) is 0, and the oxidation state of Li+ is +1. Oxidation numberThe oxidation number is a positive or negative number assigned to an atom to indicate its degree of oxidation or reduction. The term oxidation state is often used interchangeably with oxidation number. It is determined according to a set of rules based on the fact that electron pairs in covalent bonds belong exclusively to the more electronegative element. In most compounds, lithium has an oxidation number of +1 because it tends to lose an electron in order to achieve a stable electronic configuration.
You may like
Electrochemical Reactivity of 1,3-Dimethylbarbituric Acid as a Carbon-Centered Nucleophile
Dec 5, 2025
Synthesis of Lambda Cyhalotric Acid
Dec 5, 2025