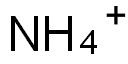The role of NH4+ flame test
Description
NH4+ (Ammonium) is a colorless cation and gives colorless salts with colorless anions. NH4+ is only present in an acidic medium. In an alkali medium, the following reaction occurs:
NH4+ + OH-→NH3 + H2O
The ionic radius of NH4+ is very close to K+. Hence, NH4+ gives most of the reactions that K+ gives.
Define
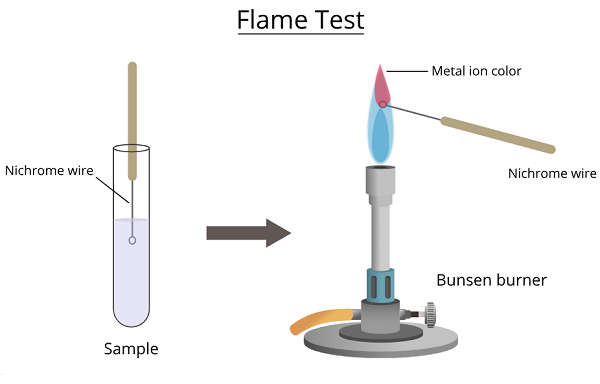
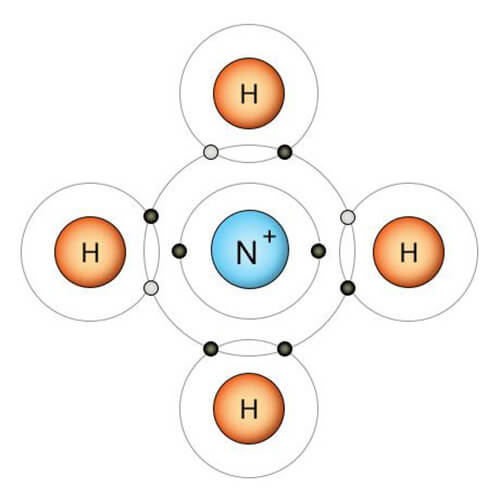
The NH4+ flame test, also known as the Ammonium flame test, is performed in a laboratory setting to identify the presence of ammonium ions (NH4+) in a given substance or solution. The test utilizes the characteristic behavior of ammonium salts in heat. The ammonium salt decomposes by subjecting the substance to a flame, releasing ammonia gas (NH3) and water vapor (H2O). The ammonia gas reacts with the hydrogen ions in the flame, resulting in a characteristic yellow-orange coloration. Vapors of NH4 salts also give a yellow color in the flame test for a short time. It can be mistaken for sodium. So, if there is NH4 in the sample, it must be removed.
Shortage
This test is a simple and quick qualitative method used primarily for educational and preliminary identification purposes in chemistry labs. It helps confirm the presence of ammonium ions, which can be an essential factor in analyzing and characterizing compounds or mixtures. However, it is important to note that the NH4 flame test alone cannot identify a specific compound, as other elements or compounds may also produce similar colored flames. Therefore, it is often performed in conjunction with other tests and analytical techniques for a more comprehensive analysis.