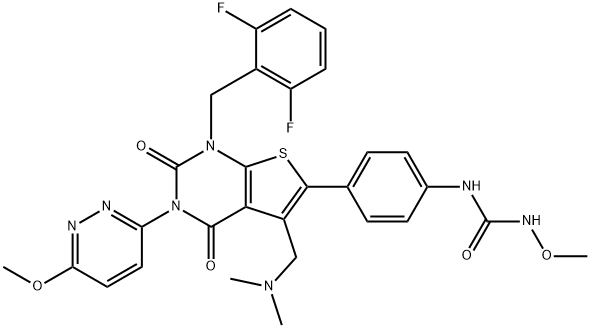
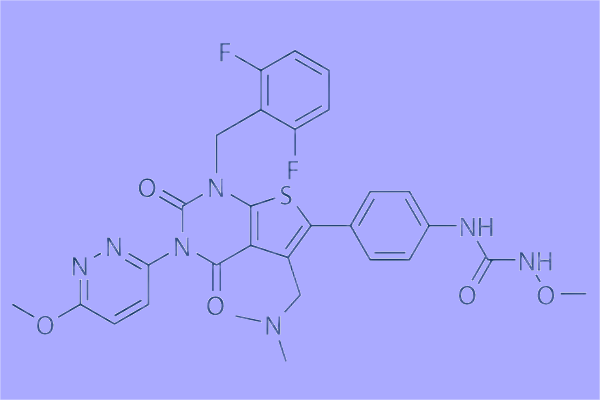
How to synthesis Relugolix?
Description
Relugolix is a nonpeptide gonadotropin-releasing hormone (GnRH) receptor antagonist discovered by Takeda Pharmaceuticals and developed by Myovant Sciences. Relugolix was recently approved for marketing in Japan as a treatment for symptoms associated with uterine fibroids, and studies evaluating the efficacy of the drug as a treatment for endometriosis-associated pain and prostate cancer are currently underway[1].
Relugolix is a well-tolerated, safe drug; it is effective in inducing a dose-dependent decrease in menstrual blood loss, with a faster reduction of heavy menstrual bleeding (HMB) and a more significant shrinkage in fibroid volume compared to the current standard of GnRH agonist treatment[2].
Synthesis method
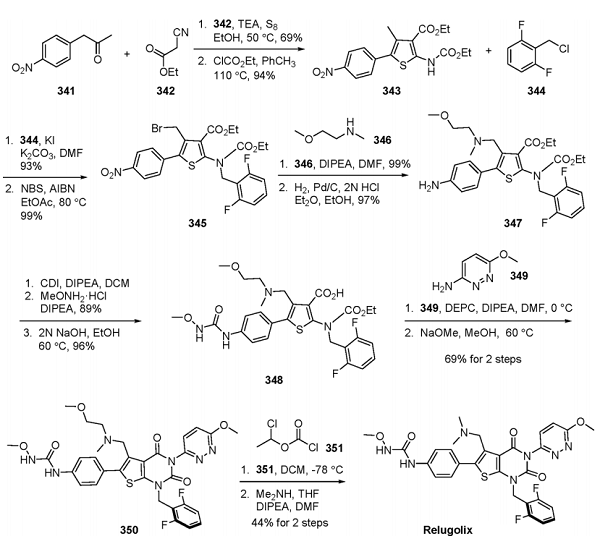
Although no explicit large-scale drug synthesis has been reported to date, researchers at Takeda disclosed a discovery chemistry route to relugolix described in Scheme 57. The reaction of ketone 341 and ethyl cyanoacetate (342) in the presence of triethylamine and sulfur resulted in the formation of an aminothiophene, a process known as the Gewald aminothiophene synthesis[3]. The corresponding amino group was protected as the ethyl carbamate in 94% yield to generate thiophene 343. This intermediate underwent substitution with benzyl chloride 344 and was then subjected to radical bromination conditions (NBS, AIBN) to give rise to benzyl bromide 345 in 93% yield over two steps. Displacement of the bromide with amine 346 followed by reduction of the nitroarene gave aniline 347 in 96% yield over two steps. This aniline was reacted with CDI, and the resulting imidazolide was further treated with N-methoxyamine to arrive at urea 348. At this stage, a cyclization reaction mediated by diethylpyrocarbonate (DEPC) in the presence of aminopyridine 349 followed by exposure to sodium methoxide generated thymine derivative 350. Hoffman conditions using chloroformate 351 were employed to quaternate the essential center within 350, which dimethylamine displaced to give relugolix in 44% yield over two steps.
References
[1] Markham, Anthony. “Relugolix: First Global Approval.” Drugs 79 6 (2019): 675–679.
[2] Kamal Kant Sahu. “Relugolix in the management of prostate cancer.” Expert Review of Anticancer Therapy (2022): 891–902.
[3] Andrew C. Flick. “Synthetic Approaches to the New Drugs Approved during 2019.” Journal of Medicinal Chemistry 64 7 (2021): 3604–3657.
You may like
Related articles And Qustion
See also
Lastest Price from Relugolix manufacturers
US $0.00-0.00/KG2025-12-01
- CAS:
- 737789-87-6
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $0.00-0.00/g2025-09-10
- CAS:
- 737789-87-6
- Min. Order:
- 10g
- Purity:
- 99%
- Supply Ability:
- 10kg