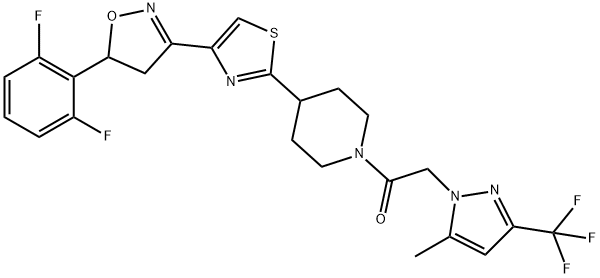
How is Oxathiapiprolin synthesised?
Synthesis of Oxathiapiprolin
Oxathiapiprolin is synthesised using 4- cyanopiperidine as a raw material by chemical reaction. The specific synthesis steps are as follows:
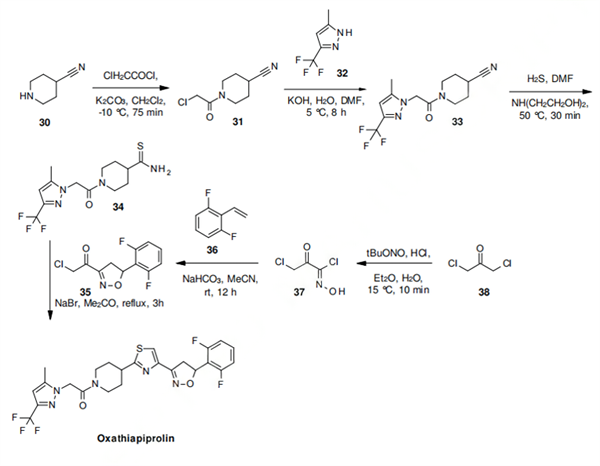
The synthesis of oxathiapiprolin begins with the acylation of 4- cyanopiperidine (30) to the chloroacetyl derivative 31, which is then alkylated on the ring nitrogen of 5-methyl-3-trifluoromethylpyrazole (32) to yield the pyrazole acetamide 33. The conversion of the nitrile function in 33 to a thioamide affords the important building block 34, which is then reacted with the chloroacetylisoxazoline derivative 35 to yield oxathiapiprolin.
The required intermediate 35 is readily obtained in just two steps from 1,3-dichloroacetone (38) by oximation to the carboximidoyl chloride 37 followed by 1,3-dipolar cycloaddition with 2,6- difluorostyrene (36).
Introduction of Oxathiapiprolin
Oxathiapiprolin was announced by Du Pont in 2012. This compound belongs to the new class of piperidinyl thiazole isoxazolines and selectively controls plant pathogens of the Oomycete genus by inhibition of oxysterol-binding protein, a mode of action which doesn't exist amongst commercialized fungicides. Whilst established market products need 100 g or more per hectare to efficiently control Phytophthora infestans, the causal agent of potato late blight, and Plasmopara viticola, responsible for grape downy mildew, oxathiapiprolin achieves the same effect with 10 – 20 g/ha, a tenth of the typical use rate. Therefore, the arrival of oxathiapiprolin on the fungicide market will set a new standard in the control of downy mildew diseases. Oxathiapiprolin has also been described to be efficacious against Phytophthora capsici in bell pepper and against black shank in tobacco, caused by Phytophthora nicotianae.