Mandestrobin: Synthesis and Introduction
Synthesis of Mandestrobin
Mandestrobin is synthesised using 2,5-dimethylphenol as a raw material by chemical reaction. The specific synthesis steps are as follows:
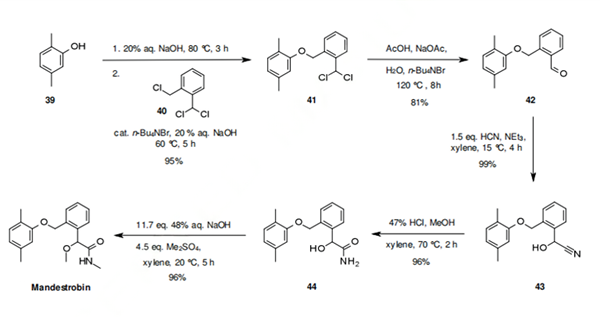
The synthesis of mandestrobin begins with the alkylation of 2,5-dimethylphenol (39) with 2- (chloromethyl)benzal chloride (40), to give the intermediate, which upon hydrolysis provides the aldehyde 42. Aldehyde 42 is converted into the cyanohydrine 43 by reaction with cyanide. Two process patents have been filed for this step; The latest one, not depicted in the scheme vide infra involves the following conditions: [HCN, n-Bu4NBr, xylene, MeOH, H2O, rt → 10°C then AcOH, H2O, 0.5 h, 10 °C; 10 °C, pH 7.76; 10 °C, pH 7.37; 3 h, 10 °C]. Finally, mandestrobin is generated by hydrolysis of the nitrile group, followed by a bis-alkylation with methyl sulfate.
Introduction of Mandestrobin
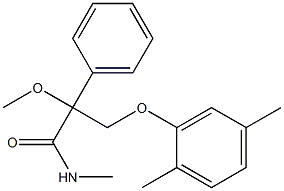
Mandestrobin is a new strobilurin registered by Sumitomo Chemical Company. The strobilurins are a very well-known class of fungicides. They reached the market in the early 90's and major crop protection companies have several of them in their business portfolios as such compounds tend to have an extremely large spectrum of efficacy. Strobilurins act as antifungal agents by inhibiting the complex III of the respiration cycle at the Qo site. There have been several new strobilurins registered recently, but we are only discussing mandestrobin in this review as it possesses a new toxophore, a 2-methoxy-N-methyl-acetamide.